For years now, many environmentalists and consumer advocates have called on the government to ban a chemical called bisphenol A or BPA. It’s found in various plastics like polycarbonate and the epoxy resins that line the inside of tin cans, and so it leaches into various foods (especially canned foods) at low concentrations.
The FDA and the EU have concluded several times it was safe at the levels present in food. In response, some folks accused FDA of surrendering to industry pressure. New York Times columnist Nicholas Kristof claimed that “Big Chem” was blocking action by FDA and deliberately exposing Americans to dangerous chemicals. “In my reporting around the world,” Kristof wrote, “I’ve come to terms with threats from warlords, bandits and tarantulas.” But chemicals like BPA scared him much more than any of those.
I imagine it was hard for many like Kristof to understand what FDA was thinking. Here was a chemical that could mimic the effects of estrogen. Various studies had found BPA at high concentrations in blood and urine samples from people in the general population. Why wasn’t it banned already? Clearly industry pressure on FDA must be to blame.
And yet, FDA’s decision was nowhere near as senseless as it seemed to Kristof. FDA had solid reasons to consider it safe at the levels found in food — and no, those reasons had nothing to do with industry pressure (at least not so far as I can tell). The earlier studies that found BPA at high concentrations in human blood and urine may have suffered from a serious defect. This part is kind of interesting, so let me try and explain how I think FDA was probably looking at this. As always, please be aware I’m not a doctor and this is my personal blog (and therefore just my personal opinion). With that said, here we go.
If you look at the structure of BPA (see below), you’ll find most of it is hydrocarbon; carbon atoms bonded to each other and to hydrogen atoms. Carbon and hydrogen share electrons fairly equally. They can’t form weak interactions with water molecules called hydrogen bonds. True, the two OH groups at the end share electrons much more unequally and can form hydrogen bonds, but they’re a relatively small fraction of the molecule’s surface, and they’re not acidic enough to give away a hydrogen ion and wind up with a negative charge at the pH ~7.4 of your blood. Since most of BPA’s surface is greasy hydrocarbon, BPA dissolves poorly in water.
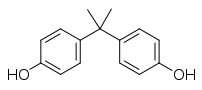
The two OH groups at the end are electron rich and can react with highly reactive electron-starved carbon atoms in molecules like phosgene, which is how BPA is used to make polymers. React BPA with something like phosgene and you end up with long long chains that tangle and stick together to form hard plastics like polycarbonate. Only trouble is, not all of the BPA gets used up in the reaction. A little BPA is left lingering in the plastic. And yeah, it has poor solubility in water, but it can still get into solution in water at concentrations in the low parts per million range. Milk and greasy foods are much better at dissolving BPA. So a little of that trace BPA left lingering in the plastic can leach into the food. Data from a range of studies looking at concentrations of BPA in food and in human blood/urine samples suggests the average adult consumes significantly less than 1 millionth of a gram of BPA per kilogram of body weight per day. In other words, if you weigh about 70 kilograms, your daily consumption given a typical diet is probably significantly less than 70 millionths of a gram of BPA. (A gram is about 0.035 of an ounce.)
Inside the human body BPA gets absorbed, but less than 1% of the BPA actually makes it into your circulation intact, and your body gets rid of even that 1% very quickly. Based on numerous studies and in-house government research, FDA found that bisphenol A was rapidly metabolized by the liver on its way into the body, so much so that concentrations in blood samples taken from volunteers after consuming food containing BPA were so low they were not detectable. The liver alters most of the incoming BPA by attaching a large sugar molecule called glucuronic acid to one of those two OH groups; some of the BPA gets a sulfate group attached to an OH group instead. Either alteration makes the BPA both harmless and highly water-soluble so the altered BPA is excreted rapidly in your urine. The altered BPA has a half-life in the human body less than 6 hours; the unaltered chemical has a half-life less than two hours. The resulting concentrations in the human bloodstream were far too low to mimic estrogen. BPA is only a weak estrogen mimic; it binds to estrogen receptors thousands of times less tightly than estradiol does, so you’d need much higher concentrations of BPA than what you see to cause a problem. Rats fed hundreds of times more BPA than what humans typically consume didn’t have any problems.
But what about the studies that found BPA at high concentrations in blood from people in the general population? It’s difficult to know for certain why their results were higher, but as one toxicologist pointed out in an interview with NPR (and as FDA also noted in their review), it’s likely contamination was to blame. As an analytical chemist who works in the pharma industry, this is an issue I’m only too familiar with. The role of analytical chemistry is to figure out 1) what chemicals are in here, 2) how much of them and 3) what’ll happen to them if they sit around (will they get metabolized, oxidized, break down etc). If you’re trying to measure concentrations of a chemical like BPA in blood samples, you have to be really careful, because BPA is found in some plastic used in the lab; plastic tubes and so forth. To make matters worse, the doctor’s office/lab that collected the blood samples quite possibly collected and/or stored them in plastic syringes or vials that contained BPA. If they did that, well, then the blood samples will almost undoubtedly have picked up some BPA by the time they reached the analytical lab — BPA that wasn’t there to begin with.
As noted in the NPR interview, the government toxicologists said they initially ran into contamination problems when they were trying to measure concentrations of BPA in blood. Once they got rid of the contamination problem, they found that BPA generally wasn’t present in blood samples from human volunteers at detectable concentrations. The labs that found BPA at high levels in human blood in earlier studies probably didn’t realize they were looking at contamination — contamination caused either by labware or (even more likely) by plastic used to collect and/or handle the blood at the lab/doctor’s office.
So FDA concluded BPA was safe at the levels found in foods for adults and children. But what about babies? Babies don’t metabolize or excrete some chemicals as rapidly as adults; their kidneys and livers don’t filter blood as rapidly. What if BPA had a longer half-life in babies? If it did, its ability to mimic estrogen at high concentrations might be more of a concern. But there wasn’t a good way to study this directly.
So FDA and the EU approached this in a different way. There’s data on metabolism of tylenol (aka paracetamol) in babies. You can use that data to figure out how good a baby’s liver probably is at glucuronidating/sulfating something like BPA. You can also look at data from baby monkeys, which are reasonably similar to humans in terms of how they metabolize these kinds of compounds (at least, more so than rats). Based on this, babies also should have negligible exposure to BPA. In pregnant women, mom’s liver takes BPA out of circulation so rapidly fetal exposure to it again should be negligible, and extrapolating based on data from animals human fetuses are also expected to be able to metabolize and eliminate BPA pretty quickly.
So what do I think about all this? My two cents would be this. It so happens I like IPAs, and IPAs happen to contain a chemical called 8-prenylnarigenen, which is one of Nature’s more potent phytoestrogens; it’s used in breast enhancement pills, in fact, although I have no idea how well it actually works in that capacity. I’m not worried about 8-PN because I know the amount of 8-PN in an IPA is so small compared to the concentrations required to cause an effect, I would need to guzzle IPAs all day in order to reach those concentrations. And yet 8-PN is almost certainly a more potent estrogen than BPA, and it almost certainly stays in the body far longer than BPA does. BPA has a half life less than 2 hours and only 1% makes it into circulation, whereas 8-PN (at least based on this study) has a half-life > 24 hours, partly because some of it gets excreted in bile and then part of what got excreted in the bile gets reabsorbed, a cycle that extends the amount of time it spends in the body.
In other words: I think if you are an adult human, BPA is not a terribly pressing concern. I can understand why people might be worried about babies and BPA, because unlike with adults you can’t study baby metabolism of BPA directly so you have to use indirect data like metabolism of tylenol or metabolism in baby monkeys. That’s something to think about. But in people of other ages, the data clearly demonstrates BPA is rapidly metabolized and excreted — so rapidly, in fact, concentrations are typically not detectable, so nowhere near — not even close — to the concentrations you would need to have an estrogen-mimicking effect. You kind of get to the point where you have to ask: how much time and energy needs to be devoted to this chemical, when the existing data makes it look like it’s reasonably safe, at least at current levels? And at what point does this become a distraction from other environmental problems?
Because, no matter what Fox News may say, we do have some pressing environmental problems. We are going to run low on fossil fuels sooner or later, we don’t yet have an economically viable replacement, and in the meantime we’re conducting a giant global experiment to determine how much temperatures will rise if we return a billion years’ worth of buried CO2 to the atmosphere. The experiment’s far from over, and so far the results don’t look good. We make lots of plastic bottles and bags, and sure they break down very slowly if you expose them to UV light and oxygen, but then we bury them in landfills where they’re exposed to neither of the above, and we have no idea how long they’re going to remain intact down there. Either that, or we litter them, and then they make their way into the ocean and break down slowly, usually starting by breaking into small pieces that may get eaten by fish. There are all kinds of species on the brink of extinction thanks to habitat destruction. In some parts of the world, we’re running low on water; in others, we have too much. And yes, there are some chemicals we’re manufacturing we’d probably be better off without; think about carbofuran or aldicarb, for example.
So no, I’m not particularly worried about BPA. Yes, that’s just my opinion, but the available data suggests to me at least (and apparently also to FDA) BPA’s unlikely to be a serious safety concern for adults, at least at the levels currently present in your food. Unfortunately, once you’ve started a controversy like this it’s difficult to rewind; people take up positions and stake their reputations on them, and raising fears is a lot easier than assuaging them. I have no doubt the BPA controversy will continue for years to come.