I feel like there’s a lot of hype about essential oils and herbs in popular culture today — check out the herbal aisle at Whole Paycheck sometime for example. They get advertised as possible cures for all kinds of disorders, often on the basis of rather thin evidence. Take, for example, this article in The Atlantic about essential oils from last week that cropped up in my facebook feed. I figured I’d blog about it, not just because it bothered me (hey, who cares) but because I think it illustrates something interesting, particularly if you don’t work in medicine and don’t spend a lot of time thinking about what properties make a molecule into a useful drug. (I mean, come on. Who doesn’t spend all their time thinking about that kind of stuff?)
The author of this article makes three main arguments. First, she claims that adding essential oils from herbs (e.g. oregano oil) to animal feed could help keep farm animals healthy and presents some evidence to back that up (farmers who are doing this now etc). I don’t know enough about chicken farming to know how practical this is, although anything that would reduce antibiotic use in agriculture is great so far as I’m concerned, because there’s evidence to suggest antibiotic use in farming accelerates the emergence of antibiotic resistant bugs in humans. So this part was interesting and it’s what got me to read the article in the first place, although again, I can’t really comment on how practical it might be.
Second, the author argues that some of these essential oils — specifically tea tree oil — make good antiseptics so they’d be useful in hand sanitizers. And yeah, tea tree oil is used as an antiseptic in various personal care products already, so I can’t really disagree with that.
Finally, the author argues that because oregano oil and certain other essential oils kill bacteria in a petri dish, you might be able to treat infections with them in humans and animals. She claims this needs and deserves more investigation but the pharma industry isn’t interested because there’s not enough money in it, and hence these “new antibiotics” have so far been ignored, although new research is turning all this around. It’s this third claim that doesn’t make sense to me.
First point I want to make: chemicals from these essential oils are already used in many consumer products. Thymol is one of the two main chemicals that make up oregano oil, for example, and it’s a common ingredient in supermarket-brand mouthwashes like the one I use. But the properties you need in a mouthwash and in a drug are not the same. And in fact, the available evidence (even some of the evidence cited in the article) suggests it’s unlikely that chemicals like the thymol and carvacrol in oregano oil would make useful antibiotic drugs. The reason why is sort of interesting, because it illustrates what kinds of properties make a molecule useful as a drug. So let me explain. And as always I’ll keep this pretty simple because that’s what I (try to) do.
——–
If you’ve read this blog before, you’ve heard me say it a million times but I’m going to keep saying it again and again. Everything is toxic; the only difference is that some things are toxic at a much lower dose. And that’s not just true for humans. It’s also true for bacteria in a petri dish. You can kill them with salt if you dump enough of it in there. But that doesn’t mean salt is a useful antibiotic: the dose of salt it would take to kill the bacteria causing your UTI is very similar to the dose of salt it would take to kill you.
Likewise bleach. In a petri dish, bleach is one of the most effective bug-killers known to humankind. It kills any and every bacteria that’s ever been discovered, reliably and efficiently. And it kills bacteria at a much lower dose than say salt for example. So why don’t we use bleach as an antibiotic? Because the dose of bleach that would kill the bacteria causing your UTI is very similar to the dose it would take to kill you.
You get the idea. For something to be an antibiotic, the dose that will kill the bugs has to be much smaller than the dose that will kill you. That’s why bleach and salt won’t work. But how can we find chemicals that will?
If you want to figure out whether a chemical could be an antibiotic, start with these two questions.
1) What concentration of the chemical does it take to stop a given strain of bacteria from growing? This concentration is called the MIC (minimum inhibitory concentration) in pharma-speak, and it’s usually measured in micrograms of drug per milliliter of broth (or blood or whatever the bugs are growing in) although that’s basically the same thing as ppm and more people are used to thinking in terms of ppm so that’s what I’ll use here.
2) Could we give you a dose that would cause concentrations of this chemical in your bloodstream to stay above MIC for a reasonable length of time without causing toxic side effects? In other words, how much of the chemical do we have to pour into you to reach the MIC in your bloodstream and is that enough to harm you?
Remember, the key thing here: the chemical has to be much more toxic to the bacteria than it is to you, and it has to stay in your body long enough that bloodstream concentrations will stay above the MIC for a reasonable length of time. (There’s lots of details that go into determining whether it’s going to meet these criteria — how well the drug dissolves in water, how the drug is metabolized, etc. etc. — but these are the big picture questions).
So let’s ask these questions about the two main active ingredients in oregano oil, a pair of chemicals called carvacrol and thymol. Structures below. They look sort of similar, right? The only difference is in where the OH is attached to the ring.
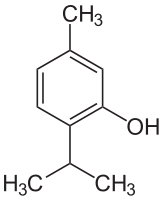

Now if you think back to previous posts, you’ll remember that groups like OH or NH2 with a “selfish” atom like nitrogen or oxygen increase water solubility. Having a charge (either plus or minus) somewhere on the molecule increases water solubility even more. These molecules, however, are mostly greasy, water-unfriendly hydrocarbon with just one OH group and that OH is nowhere near acidic enough for the molecule to have a charge anywhere near pH 7.5. So these molecules have very low water solubility, and that’s why oregano oil doesn’t mix well with water. This is something I’ll come back to in a minute. For now, though, let’s figure out whether these could be good antibiotics by thinking about our two questions, starting with what are the MICs (concentration it takes to make bugs stop growing).
You can measure MICs in a petri dish or a well plate; there’s several different ways to do it, all of them reasonably straightforward. One way is the E-test test like in this picture. You place a premanufactured strip containing preset concentrations of the antibiotic into a petri dish with the bacteria then check the dish after a preset amount of time and you’ll see something like the picture below. The # marked on the strip tells you what the concentration of the antibiotic is at that point, so that’s the concentration it takes to stop this particular strain from growing. (There are other techniques that are better for most purposes, but this I feel like this one is easiest to explain so that’s why I’m using it for an example.) The #s on the strip in the picture are in ug/mL or ppm just so you know. With MICs lower is always better (the lower the concentration of the antibiotic required to stop the bugs from growing the more potent it is.)
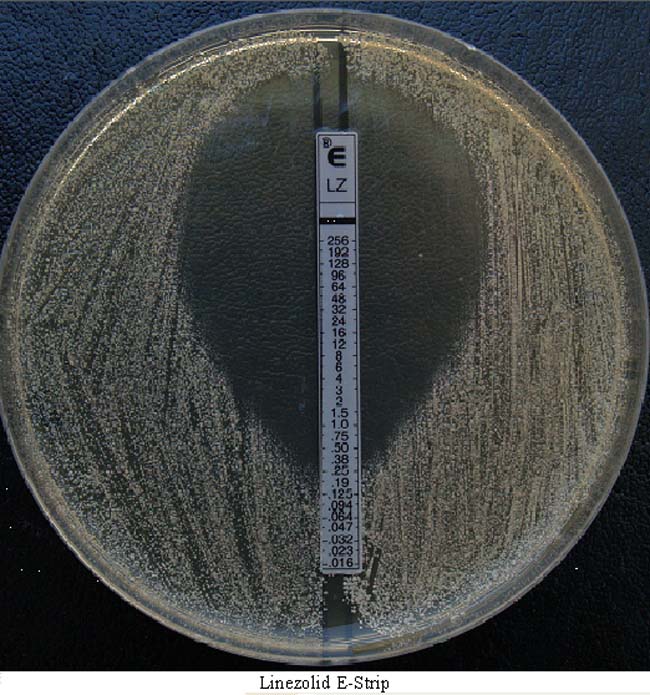
So how do thymol and carvacrol from oregano oil measure up? Not well, I’m afraid. I skimmed through a few of the papers I found on PubMed for these chemicals (there’s not a lot of research on them out there but there are some published papers) and while you can bet there’s some variation between labs and studies and techniques and so forth, from what I can find, the MICs for thymol and carvacrol against the strains that have been tested are very high. They’re very weak antibiotics.
Just to take one example, see this paper from 2001, a collaboration between academic researchers and a couple of scientists at Unilever, a company that makes a lot of the consumer products in your supermarket (detergent, soap, cosmetics, mouthwash, deodorant, etc. etc.) They tested carvacrol and thymol against an MRSA strain and got MICs of about 175 and 140 ppm respectively. To give you an idea how weak that is, consider that if a staph strain has an MIC for the drug vancomycin of 16 ppm or above, it’s said to be vancomycin-resistant.
And it gets worse. Oregano oil had an MIC of 575 ppm — probably because a substantial fraction of the oil is other chemicals that have even less activity than thymol or carvacrol. The data from this paper suggests the effects of thymol and carvacrol are additive, not synergistic, meaning that 100 ppm thymol plus 100 ppm carvacrol has about the same effect as 200 ppm of either.
But let’s try another study. This Italian study in 2004 found MICs against various staph strains of roughly 150 ppm and up. Or let’s even take one of the papers the Atlantic article linked to, this one about basil and rosemary oil. The MICs for those oils are measured in microliters per milliliter, which is parts per thousand, and they’re so high (for an MIC) when I saw them I was initially a little worried they might have been misprints.
Now these kinds of MICs are OK for a preservative or a mouthwash additive or an antiseptic; you can easily put together a product that will include thymol or carvacrol at those kinds of concentrations. My mouthwash has close to 640 ppm thymol for example in ~20% alcohol (that alcohol will help dissolve the thymol, which doesn’t dissolve well in pure water, plus at 20% alcohol’s pretty good at killing bacteria too). But if I want to use thymol or carvacrol as an antibiotic, I’m going to need to give you enough that the concentration of thymol in your blood will reach those MICs and stay above those MICs for a reasonable length of time. That’s a very high bloodstream concentration of drug to maintain, especially given that it seems your liver rapidly metabolizes these chemicals and your kidney is also filtering them steadily from your bloodstream. On top of that, these chemicals are very fat-soluble so they should quickly diffuse out of your bloodstream into your tissues (the pharma-speak jargon is high volume of distribution). Put all this together and I’d bet you my lunch the doses you’d need to reach those kinds of bloodstream concentrations are probably either worrisome or unreasonable.
Does this mean that thymol and carvacrol definitely could not work as drugs? No, I’d need more data to prove that. In particular, I’d need more complete data on how rapidly your body eliminates them (through metabolism and excretion) and MICs for more strains would be nice. And I should also point out that I just wandered through PubMed and quickly skimmed through a few papers at random here, so I don’t claim this is a full survey of the literature. But just a quick look at the available data suggests it’s unlikely these are good candidates for further investigation, at least as antibiotics. They could certainly work as preservatives or antiseptics, and the article presents some interesting data on their possible use in animal feed to help keep chickens healthy, but as antibiotics to cure an infection, well…I don’t know how that’s going to work. Moreover, most of the data I linked to above has been out there for years. It’s not new at all.
So why does this Atlantic article argue these could be “The New Antibiotics”? I think probably the folks at the Atlantic saw some papers and/or press releases claiming that “Essential Oil X Can Kill Bacteria in a Petri Dish!” and didn’t realize how little that means by itself. When you say a chemical can kill bacteria or cancer cells in a petri dish, the first question you should ask is at what concentration? because anything can kill bacteria in a petri dish if you dump enough of it in there, but it’s a question of what concentration it takes, you know. Next, you should ask is that a concentration I can reach in your bloodstream with a reasonable dose and without causing toxic side effects? because that’s what separates the drugs from the other stuff.
The other (faintly) odd thing to me about this is the end of the article. The author says that essential oils need more research because, and I quote, “they should be given the same kind of scrutiny that antibiotics haven’t been.” And this part is just simply not true. Any drug has to get approved by FDA before doctors can start prescribing it. And FDA requires reams of clinical trial data (not to mention preclinical) before they’ll approve your drug, and that data has to make sense. Extensive scrutiny is what every drug receives — has to receive — before it’s approved. So that last part makes absolutely no sense to me.