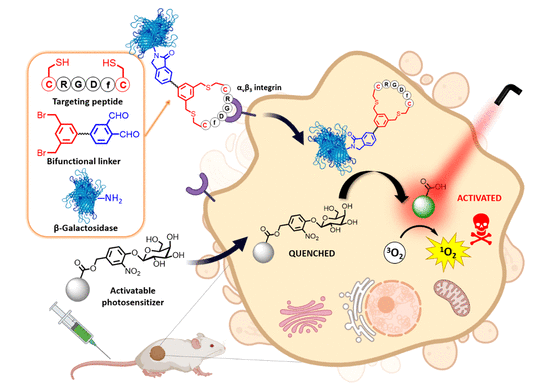
A new two-component cancer therapy uses a modified enzyme to activate a light-sensitive prodrug compound, triggering cell death exclusively in cancerous cells irradiated with a laser. The experimental treatment has already shown promise in mouse studies.
Known as photodynamic therapy, this type of treatment relies on the reaction of a photosensitive molecule with oxygen in the presence of a specific wavelength of light. The resulting reactive oxygen species rapidly cause cell death, making it a promising anticancer approach as it is a minimally invasive strategy with few side effects.
However, whilst antibodies and other ligands can help to direct photosensitiser molecules to cancer cells, the ‘always-on’ nature of their reactivity can cause problems of general toxicity as they move through the body. Two-component strategies have shown potential as they enable the photosensitiser molecule to be introduced to the body as an inactive prodrug but the need for a tumour-specific activating component has limited the development of this treatment.
A team in China has tackled this challenge head-on, modifying a lactase enzyme with tumour-targeting functional groups to ensure it is delivered exclusively to cancer cells. The chosen enzyme is not produced by humans, so when the inactive photosensitiser molecule is introduced to the body, it is only activated in the cells which contain the modified lactase – the cancer cells.
The researchers hope that their approach could be used to treat a range of cancers and be modified for use with other theranostic agents.