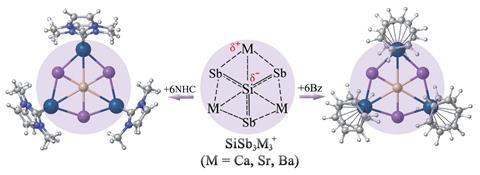
The first planar hexacoordinate silicon clusters found computationally as true global minimum forms have been reported by researchers in China, Germany and Mexico.1 The SiSb3M3+ (M = Ca, Sr, Ba) clusters, which are predicted to be both thermodynamically and kinetically stable, also remain planar when they are protected with N-heterocyclic carbene and benzene ligands that are highly stable against dissociation. This is unusual for planar hypercoordinate atom clusters, which are generally susceptible to external perturbation.
![]()
Strong covalent bonds link silicon to three antimony atoms. And a combination of ionic and weaker covalent interactions link silicon to three M atoms. The Si–Sb bonds are very strong and the alkaline earth metals interact with the silicon centre by employing their d-orbitals. These d-orbital interactions are key to the clusters’ stability, with both electrostatic and covalent interactions in the Si–M bonds being attractive.
The researchers suggest the bare clusters should be possible to detect in the gas phase, and that the ligand-protected clusters SiSb3M3(NHC)6+ and SiSb3M3(Bz)6+ could possibly be synthesised on a large scale. While there have been no experimental reports of planar hexacoordinate silicon clusters so far, recent large-scale syntheses of planar tetracoordinate silicon have attracted scientists’ attention.2,3
References
1 C Chen et al, Chem. Sci., 2022, DOI: 10.1039/d2sc01761j (This article is open access.)
2 P Ghana et al, J. Am. Chem. Soc., 2021, 143, 420 (DOI: 10.1021/jacs.0c11628)
3 F Ebner and L Greb, Chem, 2021, 7, 2151 (DOI: 10.1016/j.chempr.2021.05.002)