Researchers have developed an innovative, energy efficient process to purify the three xylene isomers – ortho-, meta– and para-xylene. The method makes use of manganese coordination polymers, a stable and low-cost material that selectively adsorbs, recognises and separates the three molecules, in both vapour and liquid phases.
The three xylene isomers ‘have very similar molecular size, shape and physical properties, including boiling points’, explains co-lead author Jing Li from Rutgers University, US. Therefore, separating them is usually extremely complicated. The boiling points of ortho-, meta– and para-xylene fit within a small range of temperatures – from 138.4 to 144.5°C – which makes distillation difficult and energy intensive. Adsorbents are also average at separation. The three isomers have ‘similar affinity towards conventional adsorbents like zeolites, leading to low selectivity’, adds Li. ‘Typical industrial [systems need] 24 separation columns to yield para-xylene with the required purity,’ she explains.
Nevertheless, the team started studying one-dimensional coordination polymers – adsorbent materials with higher structural flexibility than other porous products, such as zeolites and metal–organic frameworks (MOFs). In fact, xylene shouldn’t fit inside the pores of the manganese coordination polymer. But, when exposed to xylene mixtures, its structure swells – the distance between the polymer chains grows. ‘[It] shows a noticeable swelling effect,’ says Li, which enables the absorption and separation of the xylene isomers.
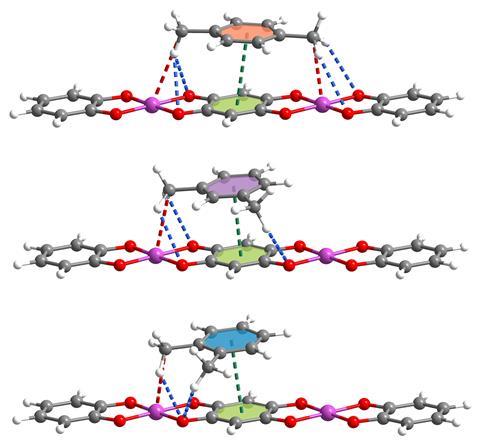
‘The interchain distance, [namely] the pore size, of this stacked polymer changes with temperature,’ explains Niveen Khashab, an expert in functional materials at King Abdullah University of Science and Technology in Saudi Arabia. ‘Such [an] extraordinary property [provides] excellent selectivity to discriminate the three xylene isomers,’ she adds. In particular, para-xylene fits these cavities very well, thus showcases a stronger affinity. ‘Para-xylene’s ratio is more appropriate for this space, [which] further leads to stronger hydrogen bonding and dipole–dipole interactions’ with the manganese coordination polymer, explains Khashab.
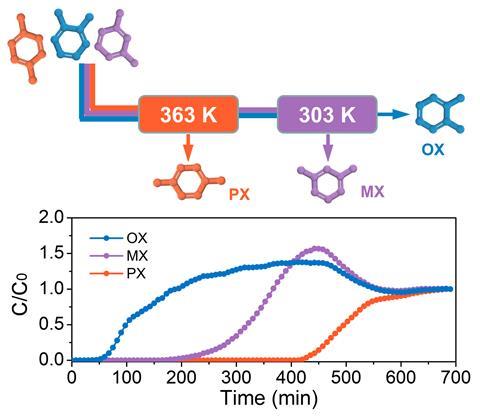
In this process, temperature is key. ‘At relatively high temperatures, such as 120°C, [it] extracts para-xylene from a xylenes mixture,’ explains Li. Then, lowering the temperature to 60°C enables the separation of the remaining mixture of ortho– and meta-xylene. It all works ‘on an industrialised separation model, [with] simulated moving beds’, says Khashab. In this setup, xylene blends break through columns filled with the adsorbent materials.
Commercially, the selectivity towards para-xylene is highly valued, as this isomer is the main precursor of terephthalic acid, which is used to make plastics, explains Mohammed Khan, an executive who’s worked for several chemical companies. The process is also interesting because it avoids distillation, he says, and could become industrially relevant if it demonstrates scalability, as well as potential uses for the other isolated isomers. Both Li and Khashab believe the process is easily scalable. ‘Simulated moving beds is an efficient, reliable and mature technology,’ explains Li. Moreover, the manganese polymer works ‘in large scale, moderate conditions and relatively low cost’, she adds.
Currently around 75% of the world’s para-xylene separation uses selective adsorption, estimates Khashab. Now, the manganese coordination polymer needs to demonstrate its long-term capacity, stability and recyclability. But it could conquer the market, because ‘it’s more energy efficient and, consequently, more environmentally friendly than currently used processes’, she says. ‘It’s one of the most promising materials for industrial xylene separation.’