Researchers in Switzerland have synthesised lead compounds using a compact, capsule-based instrument.1 The automated process is much more efficient than manual synthetic methods so could be used to streamline drug discovery campaigns.
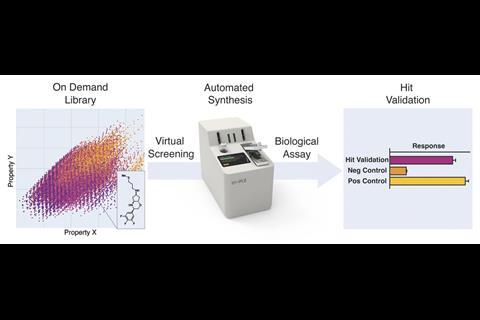
Drug discovery hinges on being able to explore vast amounts of chemical space. Virtual screening is an essential tool that allows drug discovery researchers to evaluate large numbers of compounds, including those that do not physically exist yet. Although positive hits from virtual screening studies can reduce the number of molecules that researchers need to prepare for testing, the process also generates a vast number of false positives. ‘This is the missing link in a lot of artificial intelligence-driven drug discovery programmes right now,’ says Jeffrey Bode from ETH Zurich. ‘No matter how good your computer is, you’re still not sure until you actually have the molecule in hand.’
In 2021, a team around Bode and Benedikt Wanner from Synple reported how they had developed an instrument that used prepacked reagent cartridges for synthesising and isolating organic compounds with minimal user input.2 Their latest study investigated if this instrument could be used to automatically prepare drug-like compounds from virtual screening hits using only commercially available building blocks.
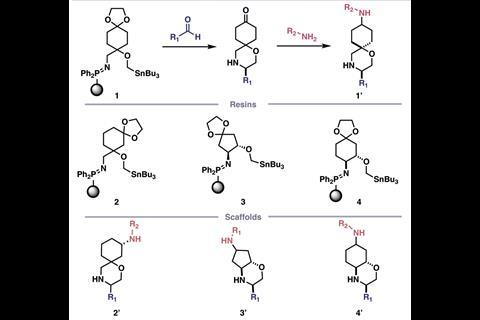
They began by building a virtual library of over 7 trillion molecules. This was divided into sub-libraries of reaction types that can be performed by the console using different reagent capsules, including reductive aminations, amide bond formations, Mitsonobu and stannyl amine protocol (SnAP) chemistry, and deprotections.
Sample molecules from each sub-library were screened against a database of commercially available compounds to investigate their structural similarities. Just one sub-library, the iSnAP library, proved structurally unique to the database and worthy of further investigation. The iSnAP sub-library consisted of spirocyclic or fused morpholine N-heterocyclic compounds that that can be prepared from commercial aldehydes and amines with capsule-based iminophosphorane-tin (iSnAP) reagents.
Bode says ‘having a lot of molecules with bad properties doesn’t get you a drug.’ ‘However, investigating fewer molecules, all with interesting structures and good physicochemical properties, is more likely to lead to a compound that you can further develop,’ and indeed, physicochemical analysis of the iSnAP sub-library showed good coverage of drug-like space.
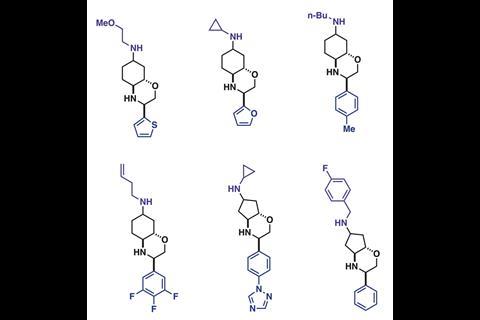
The researchers sent the iSnAP sub-library to various collaborators for virtual screening, where 20 compounds of interest against 12 target microbial organisms were identified. These compounds, plus 21 other examples from the iSnAP library, were then synthesised in a fully automated manner using the capsule-based console. These compounds could also be produced as separable diastereomeric mixtures, where the relative stereochemistry could be assigned using simple NMR spectroscopic experiments, further streamlining preparation of the screening hits.
While the synthesised hit compounds showed just modest activity when screened in vivo against 12 target organisms, the research led by Bode and Wanner demonstrates that desirable, drug-like molecules can be assembled from commercial building blocks in a fully automated fashion.
‘Tin chemistry is seldom used in the pharmaceutical industry due to toxicity concerns,’ comments Rachel Grainger, an automation lead at Astex Pharmaceuticals in the UK. ‘However, there could be increased uptake of this method for heterocycle synthesis in industrial settings, if the automated reactor can limit the user’s exposure to organotin reagents.’
The researchers calculated a 10-fold increase in user efficiency via their automated route by comparing the number of user hours needed per molecule to the corresponding manual synthetic routes.
Grainger adds that this platform could ‘make rapid compound synthesis and purification accessible to non-specialists or in settings where lab space is limited for synthesis, eg in biology labs or research hospitals.’