A new type of dual-backbone ‘ladderphane’ polymer that combines extremely high thermal conductivity with very low electrical conductivity has been reported by researchers in China and the US.1 The team believe their polymer could help to prevent overheating in the growing number of high-power applications such as vehicles and aerospace systems that use electricity as an energy source. But experts in the field are divided on the publication in Nature, and question whether the material is potentially useful or even whether the reported structure is correct.
Dielectric polymers are useful for capacitive charge storage as they are flexible, cheap and can sustain high electric fields before an arc discharge occurs and the material breaks down. At high power, however, significant heat is generated. If this heat is not dissipated, it can degrade the polymer and potentially lead to dangerous ‘thermal runaway’ – when the dielectric begins to break down, generating a positive feedback cycle of ever-greater heat production. Unfortunately, most polymers that conduct heat well also have significant electrical conductivity, which wastes energy.
In metals the principal carriers of heat are electrons, whereas in polymers heat mainly moves as quantised vibrations called phonons. The mean free path of a phonon in a polymer is normally small, giving polymers low thermal conductivity. The researchers wondered whether highly-ordered polymers called ladderphanes, comprising two polymeric carbon backbones joined by regular linking segments, might conduct phonons better. Some ladderphanes, however, have higher electrical conductivity than some other polymers.
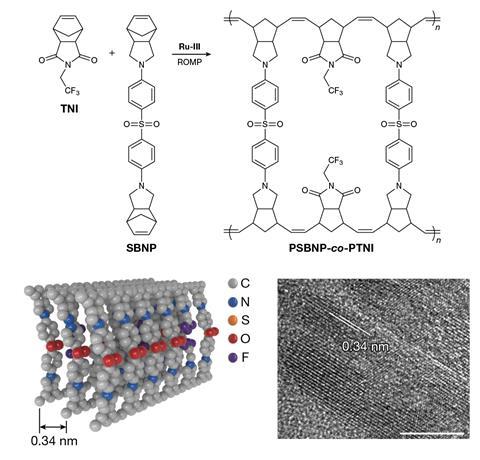
The researchers therefore designed ladderphane copolymers using two monomers, a pyrrolidine and an imide, that self-assembled into identical highly-ordered, parallel copolymer arrays. The introduction of the imide, which has a much greater electron affinity than pyrrolidine, was intended to create ‘charge traps’, preventing electrons from escaping the material. The researchers report that the resulting copolymers combine very low electrical conductivities, record-high thermal conductivities, very high electrical breakdown voltages and the useful ability to self-heal rather than failing catastrophically.
Tien-Yah Luh at the National Taiwan University in Taipei, who headed the research group that first reported ladderphanes in 2009,2 is impressed. ‘It’s really wonderful that ladderphanes can be used in these kinds of applications – it can increase the [charge/discharge] efficiency by 40% in comparison with existing systems. A particularly interesting point here is that, as you increase the temperature, the conductivity becomes lower.’
Gregory Sotzing at the University of Connecticut in the US – part of the team that previously held the charge/discharge efficiency record3 – is unconvinced, however. ‘I see that they have good measurements, but I don’t know what they’re measuring,’ he says. ‘They’re a little bit better than us [in efficiency], and the question is why. It could just be a smaller electrode area size that means you get rid of more of the morphological defects in your sample… They’ve tied this right to structure.’ The proposed structure comprises two parallel backbones, each comprising a series of double bonds – all in the trans configuration – with linking segments pointing inwards at right angles, and bonding to form a DNA-like double helix.
Luh sees no cause for concern, and says that ‘based on the spectroscopic evidence [the researchers provide 1H and 13C NMR as well as Raman and IR spectroscopy], this is very comparable to the compounds we have studied in our laboratories.’ He also notes that research from his laboratory has shown that the choice of catalyst can control the stereochemistry of the backbone.
Sotzing argues that the formation of such a regular structure, with all bonds taking the same isomeric configuration, in a copolymer would be extraordinary, however, and that extraordinary evidence to support such a claim is lacking. As just one example, Sotzing – an NMR expert – notes that the researchers cite as proof the bonds are all in the trans configuration in the ladderphane and the fact that a second peak seen in the analysis of the single-chain molecule is no longer present, but do not further investigate an asymmetric shoulder in the remaining peak. ‘I think it’s two peaks,’ he says. ‘[That would mean] you have a mixture of cis and trans – and then you’re really in trouble.’ If they do not have long chain ladderphanes but cross-linked polymers, he says, they would be prone to cracking when rolled into functional capacitors.
The researchers who created the new ladderphane respond that other information such as Raman spectroscopy supports their proposed structure. They also add that measurements of the molecular weight and mechanical properties of the polymer support their contention that it does indeed form a long chain ladderphane.
Jeremiah Johnson of Massachusetts Institute of Technology is also doubtful though. ‘The chemistry in this paper does not make sense,’ he says. ‘The “ladderphane” structures they draw are very unrealistic. I looked through their characterisation data, and they do not provide convincing evidence that the material has the chemical structure they show. Maybe the properties they get are real (I believe they likely are since the PI of the group [Qing Wang] is an expert on the properties), but they are almost certainly not coming from the structures they draw.’