
Scientists have created a guanidinium sulfate salt that can capture and store carbon dioxide at ambient pressures and temperatures, with little energy input. The strategy could change how industry captures, transports and stores the gas.
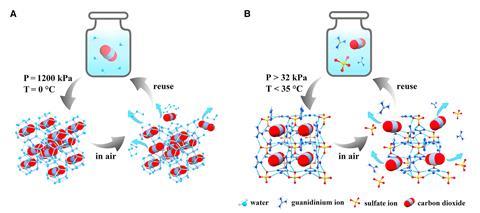
An international team of scientists charged an aqueous Gua2SO4 solution with carbon dioxide and saw a single-crystalline guanidinium sulfate-based clathrate salt form at ambient conditions. Because the solution encased carbon dioxide without forming strong bonds with the gas, researchers were able to easily reverse the process to release the gas again.
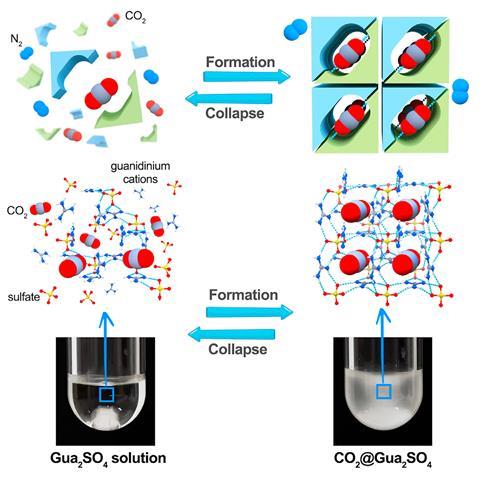
Cafer Yavuz, a researcher from King Abdullah University of Science and Technology in Saudi Arabia and one of the study authors, says that releasing trapped carbon dioxide has never been so easy. ‘All you have to do is just take this [resulting precipitate], shovel it into water, the water dissolves the salt and CO2 comes up,’ he explains. ‘It’s almost like the tablets we use to drink vitamin C. You put it in the water and bubbles [appear]. It’s CO2 coming up.’
After the carbon dioxide was released, the team found that the resulting Gua2SO4 solution was immediately ready for another cycle of carbon dioxide uptake.
Currently, the most common method of removing carbon dioxide from a gas stream involves capturing the molecule via chemisorption, usually using an amine sorbent. While this strategy is highly selective for carbon dioxide, the energy demand is high. Gua2SO4 captures the gas equally well without expending nearly as much energy.
‘It’s unique, because it has perfect selectivity like in chemisorption. But no chemical bonds are formed between CO2 and the host, like in physisorption. It’s weird. It’s in between, it’s a quasi-chemisorption quasi-physisorption state,’ adds Yavuz.
But while the salt looks promising for the carbon capture industry, Yavuz foresees other applications. Because the resulting powder is stable at ambient conditions, the clathrate could be useful to carry and store CO2, as well as capture it from industrial sources. This isn’t necessarily a problem that needs to be solved because there’s already infrastructure to transport and store CO2, but Yavuz still believes the clathrate could improve the entire carbon capture chain and cut down on costly infrastructure. ‘The best thing this powder [could be] used for is for the mobility of CO2, to carry [it] in a truck or for storing it,’ Yavuz adds.
Alexander Forse, who works on new materials for carbon capture at the University of Cambridge and was not involved in the study, says the work is impressive, and applying it to real-world scenarios will be an interesting next step. ‘This is an exciting new way to think about capturing carbon dioxide from industrial emissions like cement plants,’ he says. ‘I’m curious to see how energy-efficient this technology can be, and whether the kinetics are rapid enough to enable a practical carbon capture system.’