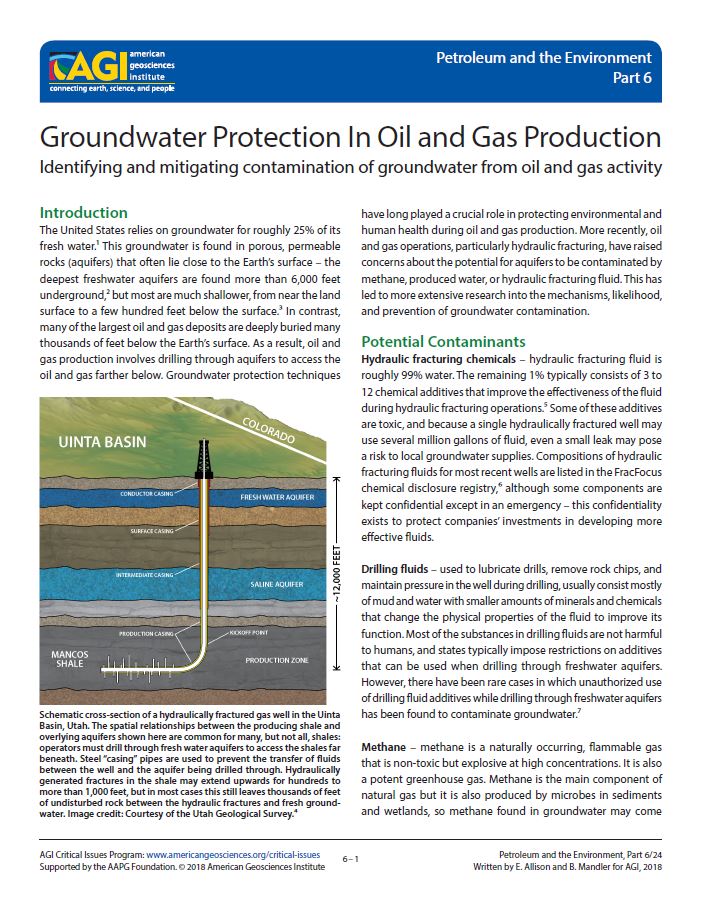
## Comprehending the Elk River Chemical Spill: Essential Information on 4-Methylcyclohexane Methanol
On January 9, 2014, a major chemical spill took place as Freedom Industries discharged a considerable quantity of 4-methylcyclohexane methanol (often abbreviated as 4-MCMH) into the Elk River in West Virginia. This hazardous incident impacted the water supply for around 300,000 individuals, prompting local officials to declare a state of emergency and cease the water supply. The incident sparked widespread anxiety regarding health and environmental safety, leaving many with inquiries about the associated risks.
This article breaks down the fundamentals of 4-MCMH — its chemical composition, potential hazardous outcomes, and what insights we can and cannot gather from its molecular arrangement.
### What Is 4-Methylcyclohexane Methanol (4-MCMH)?
4-MCMH is an organic substance frequently utilized in various industrial processes, such as coal processing. It has gained attention due to the licorice-like scent of its vapors, yet there exists limited information regarding its behavior and potential toxicity to humans.
The chemical structure of the molecule provides some hints. In basic terms, picture the carbon atoms structured in a six-member ring (a cyclohexane ring), with one carbon atom bonded to a hydroxyl group (-OH) — which contributes to the ‘methanol’ portion of its designation. It’s critical to remember that chemicals are more complex than their structural formulas might imply. While some insights can be drawn about its properties from its molecular structure, important aspects of its effects on humans and the environment remain elusive due to insufficient toxicological research.
### The Chemical Structure: 2D vs. 3D
Chemical representations are typically depicted in two dimensions, but in truth, molecules such as 4-MCMH exist in three dimensions. For a carbon atom, the bonds create a tetrahedral configuration, assisting chemists in predicting how molecules will react with various environments or biological systems.
When examining 4-MCMH, it is primarily composed of carbon-hydrogen bonds, which are non-polar, along with one polar hydroxyl (-OH) group. This combination is significant because, generally, water is better at dissolving polar substances compared to non-polar ones.
4-MCMH features a minor polar section (the -OH group), while the majority of the molecule consists of non-polar hydrocarbons. As a result, the compound exhibits poor solubility in water, and when it does dissolve, it is likely to remain at the surface rather than mix thoroughly due to being less dense than water.
### Why Did the Spill Lead to Water Contamination?
The minimal solubility of 4-MCMH in water does not imply that the spill was without noteworthy consequences. The risks of contamination stem from localized areas where concentrations could become significantly elevated. At higher concentrations, 4-MCMH poses threats to aquatic ecosystems or potential health risks for individuals exposed to the tainted water, either through accidental ingestion or skin exposure.
Moreover, due to the absence of comprehensive studies on its toxicity, health professionals and officials exhibited due diligence. Local authorities advised residents against drinking, cooking with, or bathing in the water until more definitive data emerged. Although it seemed unlikely that acute toxicity would necessitate immediate medical intervention based on limited information, the long-term effects, particularly for at-risk groups, remained uncertain.
### Is 4-MCMH Dangerous?
Is it dangerous? Possibly — though its level of danger is not clearly defined.
Based solely on the chemical structure of 4-MCMH, toxicologists can provide some reasonably informed estimates. For instance, the compound is not expected to be highly reactive, implying that it is unlikely to engage aggressively with proteins or DNA in ways that could result in immediate cellular injury. Notably, the liver, responsible for detoxifying substances, would probably metabolize 4-MCMH, enhancing its water solubility and allowing for elimination through urine.
The compound’s low solubility in water suggests the potential for accumulation in fatty tissues, raising concerns regarding chronic exposure. However, with infrequent, low-level exposure, the body is likely to metabolize it efficiently.
Toxicity studies on rats indicate that the lethal dose (LD50) for ingestion is approximately 825 milligrams of 4-MCMH for every kilogram of body weight. While this level of toxicity is not alarmingly high (humans would need to consume substantial quantities to experience fatal consequences), it does not imply that it is safe for routine exposure, especially regarding unknown long-term effects.
There’s an additional complication: The chemical remains poorly understood. The lack of thorough research means that we cannot dismiss potential harm to humans or the environment based on the currently available data. Furthermore, the report of the water turning blue-green following the spill suggests the presence of other possibly more hazardous contaminants (distinct from 4-MCMH itself) that may have been involved.