**The BPA Debate: Science, Safety, and Societal Views**
For many years, bisphenol A, widely recognized as BPA, has been at the center of contention. Environmental organizations and consumer rights advocates have persistently urged for a prohibition on this substance, asserting that it poses considerable threats to human health. The apprehensions arise from BPA’s occurrence in common plastics, like polycarbonate and the epoxy resins utilized in tin can linings, enabling it to seep into food and drinks in minute amounts. Detractors contend that the extensive use of BPA in contemporary packaging presents an intolerable risk, with some even alleging that regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Union (EU) have succumbed to industry influence to keep the substance available.
However, the pivotal question persists: Is BPA the public health menace that critics claim, or is it a mischaracterized chemical whose hazards have been largely exaggerated? In this article, we will explore the science of BPA, the rationale behind regulatory choices, and why this topic remains divisive in public discourse.
—
### **What is BPA, and Why is it Worrisome?**
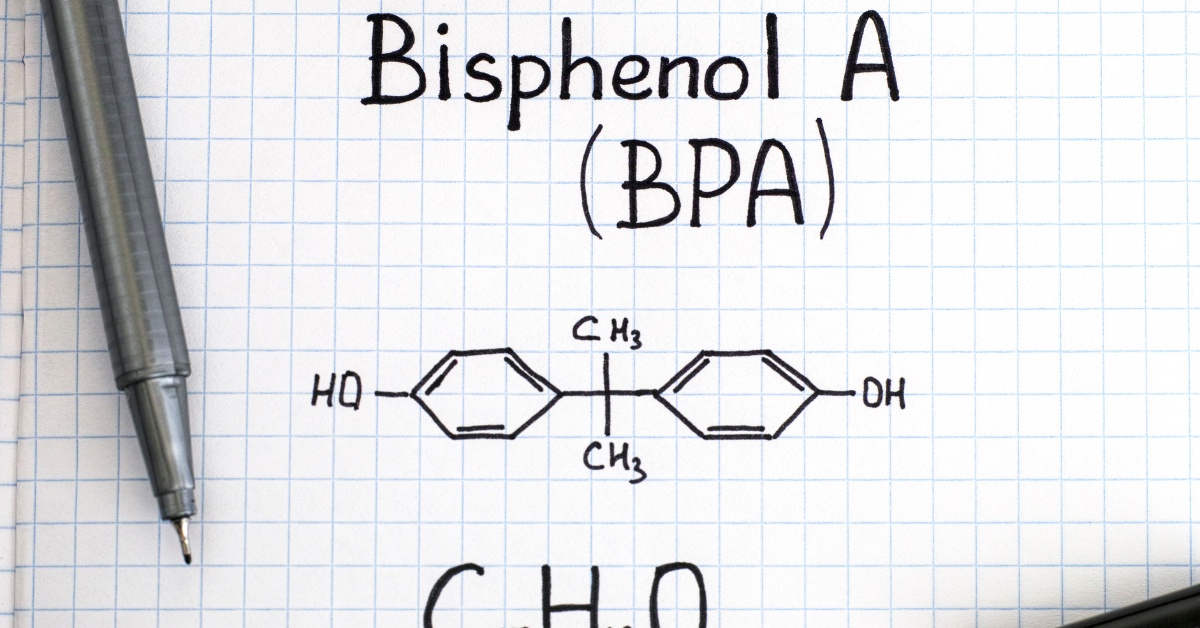
BPA is a synthetic compound chiefly employed in the production of polycarbonate plastics and epoxy resins. These substances are crucial in a vast range of items, from water bottles and food storage containers to the interiors of canned products. BPA initially drew attention due to its capacity to imitate estrogen, a hormone essential for regulating numerous bodily functions. As a “weak estrogen mimic,” BPA can attach to estrogen receptors within the body, albeit with an affinity that is thousands of times less than that of natural hormones like estradiol. This raised concerns that even low-level exposure to BPA could interfere with endocrine functions, potentially giving rise to developmental and reproductive complications.
Compounding the debate, prior research has found BPA in human blood and urine, sometimes at levels that seemed alarmingly high. These discoveries heightened the disquieting possibility that BPA is not only widespread in our surroundings but also accumulating within our bodies.
—
### **The Regulatory Conclusion: Is BPA Safe?**
The FDA and EU have conducted multiple assessments of BPA’s safety, consistently determining that BPA is safe at the concentrations typically found in food and drinks. These agencies derive their conclusions from a multitude of scientific research, incorporating both independent studies and those conducted by governmental bodies. Despite persistent pushback from advocacy groups, the available evidence indicates that human exposure to BPA is minimal and unlikely to present any significant danger.
1. **Minimal Exposure Levels**: Research indicates that the average adult ingests far less than 1 microgram of BPA for every kilogram of body weight on a daily basis. For an individual weighing 70 kilograms, this equals less than 70 micrograms per day—an amount the FDA regards as considerably beneath the “tolerable daily intake” level.
2. **Efficient Metabolism and Elimination**: When BPA enters the body, it is metabolized and expelled with exceptional speed. The liver transforms BPA into inactive and highly water-soluble metabolites, such as BPA-glucuronide, which are swiftly discarded via urine. In adults, BPA’s half-life in its active state is under two hours, which makes it exceedingly difficult for the substance to build up in the bloodstream.
3. **Difficulties in Measuring BPA Concentrations**: Earlier studies reporting elevated BPA levels in human blood have faced skepticism regarding potential contamination. BPA can be a common component of various plastics utilized in laboratory equipment, including collection vials and pipette tips. This contamination may account for why early research overestimated BPA levels, as subsequent, meticulously controlled studies frequently failed to find significant BPA concentrations in blood.
—
### **Important Considerations for At-Risk Groups**
Adults appear to metabolize and eliminate BPA so effectively that the likelihood of hormonal disruption seems minimal, but what about more vulnerable populations like infants and pregnant women? This query represents one of the foremost concerns in the BPA discourse.
Research indicates that even for infants, exposure levels are probably very low. Data from animal studies, including those with infant monkeys, suggest that the metabolic pathways for BPA become functional quite early in life. Moreover, findings indicate that pregnant women metabolize BPA so quickly that exposure to the fetus remains minimal. Nevertheless, due to the challenges inherent in directly examining BPA in infants and developing fetuses, some uncertainty still exists.
In an abundance of caution, manufacturers have voluntarily withdrawn BPA from many baby bottles, sippy cups, and infant formula packaging, particularly in regions such as the U.S. and Europe.
—
### **Big Chemical Industry, Public Anxiety, and Media Representation**
The BPA discussion has frequently involved as much about politics and public sentiment as it does about scientific inquiry. Critics, including prominent figures like New York Times columnist Nicholas Kristof, have accused the chemical sector of obscuring BPA’s risks and exercising excessive influence over regulatory agencies. Kristof has even gone so far as to assert that “Big Chem” is prioritizing profits at the expense of public health.