A New Era in Medicine: Epigenetic Editing and the Potential of Precision Gene Regulation
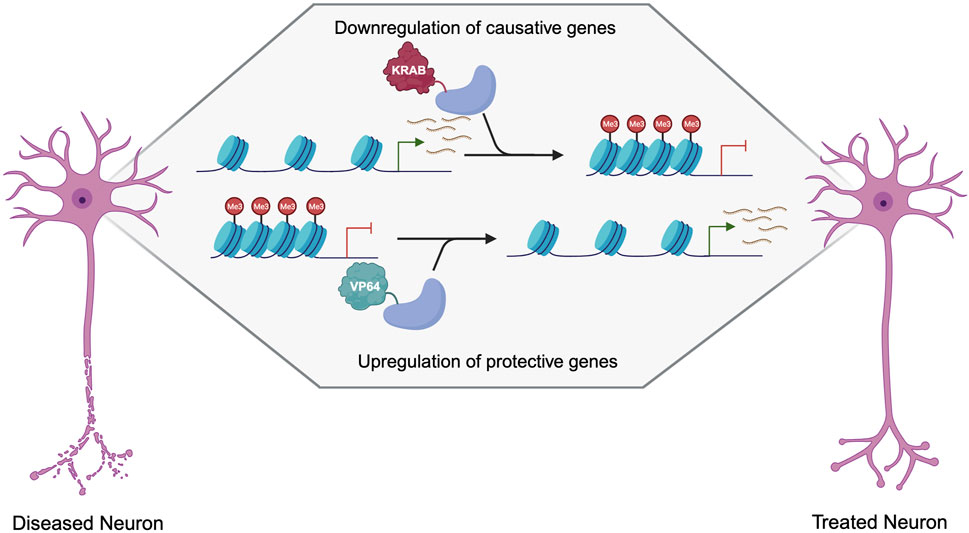
A new chapter in medical science is emerging with the rise of epigenetic editing—a remarkable technology that enables researchers to manage gene function without modifying the fundamental DNA sequence. This groundbreaking method, now advancing to human testing, has the potential to revolutionize therapies for various illnesses, including viral diseases, genetic conditions, and neurological issues.
The Shift From Gene Editing to Gene Management
Gene editing methods like CRISPR–Cas9 have dramatically altered the landscape of medicine by facilitating precise adjustments to the DNA sequence. These approaches can permanently rectify genetic mistakes by excising and repairing specific genes. In contrast, epigenetic editing adopts a different approach. Instead of rewriting the genetic instructions, it alters how genes are expressed. This modification is accomplished by adding or removing chemical markers like methyl or acetyl groups to specific DNA regions, emulating the body’s innate mechanisms of gene regulation.
These chemical alterations affect the accessibility of a gene to cellular machinery, influencing whether it is activated or inactivated and to what degree. As Derek Jantz, CSO at Tune Therapeutics, points out, the domain is reaping the rewards of significant advancements in CRISPR-targeting techniques, improved understanding of genomic regulatory elements, and more advanced delivery methods. “Everything… has converged to a point where this is now feasible,” Jantz notes.
The Safety Advantage of Epigenetic Editing
A key benefit of epigenetic editing lies in its safety profile. Since it does not involve cutting DNA, the likelihood of creating permanent mutations is significantly reduced. For instance, methylation generally requires multiple chemical interactions to substantially alter gene expression, making unintended or off-target modifications less likely to have a biological effect. Additionally, these modifications can be reversed, providing a more adjustable and responsive method of gene regulation.
New Therapeutics and Clinical Trials
Various biotech firms are now advancing the first epigenetic therapies into clinical trials:
1. Tune Therapeutics: Addressing Hepatitis B
Tune is at the forefront of the initial human trials for an epigenetic editor designed to suppress chronic hepatitis B virus (HBV). Its treatment, administered through lipid nanoparticles, utilizes mRNA to encode proteins that silence the viral genome in liver cells via methylation and chromatin modifications. Initial trials are underway in New Zealand and Hong Kong, regions with high HBV incidence. Because this treatment targets the virus’s genetic material, not that of human cells, the risk to patients is minimal. A single or limited number of doses could achieve prolonged suppression.
2. nChroma Bio: Targeting Hepatitis B and D
Boston’s nChroma Bio is also working on a lipid nanoparticle-based editor for hepatitis B and D. The company, formed from the merger of Chroma Medicine and NVelop, is implementing advanced delivery systems to improve tissue specificity. “We are observing substantial silencing of the hepatitis genome… and nearly a 99.99% decrease in hepatitis B surface antigen,” states CSO Melissa Bonner. The treatment’s capacity to accurately target the viral genome, which differs from human DNA, improves effectiveness and safety.
3. Epic Bio: Concentrating on Muscular Dystrophy
Epicrispr Biotechnologies is gearing up for a trial aimed at facioscapulohumeral muscular dystrophy (FSHD), a genetic disorder linked to the inappropriate activation of a normally dormant gene called DUX4. Epic Bio’s approach aims to restore methylation and reactivate the silencing of DUX4, reducing muscle degeneration. Distinctively, the company uses a compact CRISPR system known as CasMini, which fits into viral vectors for muscle tissue delivery—providing an elegant solution for gene targeting in intricate tissues.
4. Sangamo Therapeutics: Focusing on Neurological Disorders
Sangamo is examining neurological conditions by utilizing small zinc finger proteins—natural DNA readers—linked to repressor domains. These proteins are delivered through viral vectors to selectively silence genes like SCN9A, which encodes the sodium channel NaV1.7, crucial in pain signaling. By accurately modulating gene expression without impacting similar channels, Sangamo can more specifically alleviate chronic pain compared to conventional medications. They are also developing a pipeline for diseases such as prion disorders using their proprietary AAV vector Stac-BBB, which effectively crosses the blood–brain barrier.
Overcoming the Blood–Brain Barrier
A significant challenge in genetic therapies is effective delivery—particularly to the brain. Sangamo’s modified capsid, Stac-BBB, signifies a major advancement by facilitating the efficient transfer of therapeutic materials through the blood–brain barrier. In preclinical studies in non-human primates, Stac-BBB demonstrated 700 times greater efficacy than standard AAV9 in accessing brain tissue. This innovation paves the way for epigenetic therapies targeting complex neurological diseases, including inherited prion disorders and potentially Alzheimer’s, as evidenced by Genentech’s acquisition of the Stac-BBB system.