🌟 Revealing the Mysteries of Actinide Phi Bonds: A Significant Advancement in f-Block Chemistry
In a groundbreaking study that sheds light on a longstanding quantum enigma, researchers have uncovered the last piece in deciphering how actinides—some of the heaviest and most intricate elements in the periodic table—form bonds with ligands through an exceedingly rare interaction known as phi (φ) bonding. This discovery not only enriches our fundamental understanding of advanced chemistry but also paves the way for transformative innovations in nuclear fuel reprocessing, catalysis, and even quantum computing.
Grasping Actinides and Phi Bonds
Actinides are elements with atomic numbers ranging from 89 (actinium) to 103 (lawrencium), recognized for their radioactive characteristics and vital contributions to nuclear chemistry and energy. Among these, uranium, plutonium, americium, and curium are especially noteworthy for both their practical applications and the scientific challenges they introduce.
At the core of chemical bonding lies the overlap of atomic orbitals, which can engage in specific interactions dictated by their geometries and symmetries. Bonds like sigma (σ), pi (π), and delta (δ) are prevalent in molecular chemistry. However, the f-orbitals found in actinides are particularly adapted for an even rarer type of bonding: phi (φ) bonds, noted for their three nodal planes. These bonds are difficult to identify and have historically posed a conceptual challenge for chemists exploring the outer edges of the periodic table.
Theoretical Advancement from Los Alamos
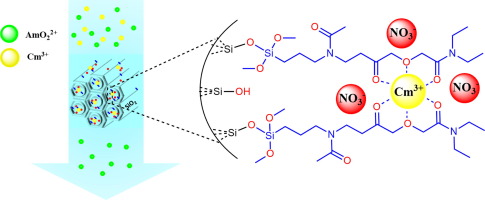
Under the guidance of Dr. Ping Yang at the Los Alamos National Laboratory, a group of chemists has employed state-of-the-art theoretical modeling to investigate actinide–ligand interactions throughout the entire array of actinides and in three distinct oxidation states. Their objective: to uncover how phi bonding shifts with the charge of the metal center.
Their results are striking. The team noted that altering the oxidation state of an actinide atom affects the strength and nature of its phi bonding interactions—specifically, the “head-to-head” nature of the orbital overlap. This enables a new degree of control over actinide-ligand bonding—a vital advancement for customizing these interactions for specific chemical objectives.
Implications: From Nuclear Recycling to Quantum Computing
The consequences of this discovery are extensive:
1. Nuclear Waste Reprocessing and Separation Chemistry:
Enhancing phi bonding control could improve selectivity in isolating minor actinides (like americium and curium) from chemically similar lanthanides, a formidable challenge in existing nuclear fuel recycling programs. As Dr. Yang notes, this could greatly boost the efficiency of spent fuel reprocessing and minimize the ecological impact of nuclear energy.
2. Redox Catalysis:
The modulation of phi bonding might facilitate the creation of innovative redox-active catalysts, designed for specific oxidation conditions. This could modernize catalytic systems that utilize f-block elements, which have been less explored than their d-block counterparts (like transition metals).
3. Quantum Information Science:
Beyond conventional chemistry, the elevated angular momentum of f-electrons makes actinides particularly appropriate for quantum computing applications. By manipulating phi bonding, researchers may one day gain precise control over electronic states, potentially allowing for new kinds of quantum bits (qubits) based on actinide atoms.
A Contribution to Fundamental Chemistry
This research has garnered acclaim from prominent figures in the field. Dr. Conrad Goodwin, an actinide researcher at the University of Manchester, praised the study as “a magnificent piece of fundamental theoretical actinide science,” likening it to seminal works by Bruce Bursten, a former president of the American Chemical Society. Goodwin underscored that the model rich in data presented by Yang’s team offers a new roadmap for experimental chemists, delineating testable predictions that could ignite a new wave of synthetic actinide chemistry.
Mapping the Future of the f-block
As scientists continue to explore the intricate behaviors of heavy elements, the capacity to manipulate nuanced bonding interactions like phi bonds signifies a fundamental shift. Not only does this validate long-held theoretical ideals, but it also equips researchers with practical tools to design novel materials, catalysts, and quantum systems grounded in f-block science.
At the farthest reaches of the periodic table, where relativity intersects with radioactivity, the revelation of how to manage phi bonds may unlock an entirely new category of chemical innovation. With actinide research receiving renewed focus, this accomplishment stands out as a cornerstone for the upcoming era of chemistry and technology.