📘 Unleashing the Power of Superatoms: Thorium Highlights Actinides
An innovative breakthrough in the realm of chemistry is redefining our comprehension of atomic interactions, magnetism, and the attributes of materials. For the first time, researchers have engineered superatoms exclusively from actinides—one of the most intricate and reactive series in the periodic table. The research team, headed by Professor Stephen Liddle at the University of Manchester, has unveiled a novel category of superatom based on a trithorium nanocluster, offering unexpected revelations regarding the bonding and magnetic characteristics of heavy metals such as thorium.
What Are Superatoms?
Superatoms are aggregations of atoms that function similarly to a singular atom of a different element altogether. First identified approximately 40 years ago, these synthetic structures can reveal electronic and chemical traits that differ from their individual constituents. One renowned example is Al₁₃—a 13-atom aluminum cluster that mirrors the chemical properties of a halogen like chlorine because of the unique arrangement and confinement of its electrons.
This alteration in behavior typically arises from quantum confinement effects and metallic bonding among the atoms. Superatoms have shown remarkable utility as nanoscale building units for the creation of novel materials and electronic devices. Previous investigations have explored superatoms containing actinides such as uranium, but only at the edges of clusters mainly composed of main-group or transition elements—not in pure actinide–actinide bonds.
Introducing Thorium Superatoms
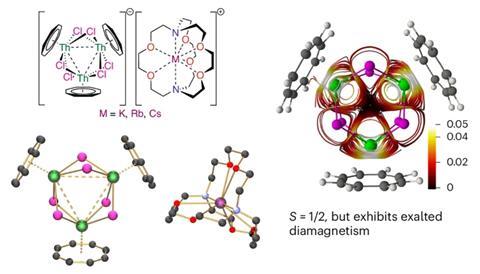
In this latest advancement, the team at the University of Manchester has synthesized a molecule that features robust bonds among three thorium atoms arranged in a ring-like trithorium cluster. Each thorium atom is stabilized by chloride ions and a wing-shaped aromatic system. This breakthrough reinforces findings from earlier studies—also spearheaded by Liddle—that actinide–actinide bonding is not just feasible but could be exceptionally stable and surprisingly robust.
The thorium trimer demonstrates aromaticity, a characteristic usually linked with carbon-based compounds like benzene. In aromatic structures, electrons are delocalized across a circular framework, contributing to both molecular stability and distinct electron behavior. Uncovering such aromaticity within an all-actinide ring is an unusual occurrence, directly confronting established beliefs regarding f-block elements.
A Magnetic Surprise: From Paramagnetic to Diamagnetic
However, the most remarkable observation may be the molecule’s magnetism. According to its electron configuration, the thorium cluster should exhibit paramagnetism—it has unpaired electrons, which generally indicates attraction to magnetic fields. Nevertheless, the molecule shows diamagnetic behavior, actively repelling magnetic fields.
This unexpected effect is being referred to as “exalted diamagnetism.” It implies that the aromatic ring-like structure amplifies diamagnetic effects beyond normal expectations, revealing a previously unexamined quantum phenomenon. Consequently, the molecule manifests the characteristics of a diamagnetic superatom. The research team suggests this behavior draws parallels to elements in Group 1 of the periodic table—specifically alkali metals—categorizing it as a kind of “super alkali-metal.”
Implications and Expert Opinions
“This finding significantly extends previous research,” remarked Thomas Albrecht, director of the Nuclear Science and Engineering Center at the Colorado School of Mines. “The initial discoveries regarding actinide bonding faced doubt, yet they have proven enduring. The diamagnetic properties of this cluster are what truly stretch the limits—it’s a significant and, to be frank, astonishing result.”
The unique traits of actinide-based superatoms pave the way for innovative uses in fields such as magnetic materials, molecular electronics, and quantum computing. Moreover, they enhance theoretical insights into actinide bonding, which is crucial for nuclear science, where managing and controlling the behaviors of actinides remains a significant challenge.
Challenges of Actinide Chemistry
Actinides are far from accommodating elements. They are radioactive, thermally unstable, and possess complex electron configurations involving f-orbitals that render their behavior especially challenging to model and forecast. Additionally, these materials frequently deteriorate rapidly under standard conditions—some disintegrating in under a week and others breaking down in solution when temperatures exceed 35°C.
Despite these hurdles, the research team’s achievements illustrate that through precise methods and innovative molecular scaffolding, stable compounds containing these heavy elements can be synthesized and examined.
Conclusion
This monumental discovery not only enhances our comprehension of actinide chemistry—it revolutionizes it. By fabricating the first superatom composed entirely of actinides, researchers have illustrated not only the unexpected strength of actinide–actinide bonds but also revealed extraordinary magnetic behaviors grounded in quantum mechanical phenomena.
The findings reaffirm that extensive exploration remains in the domain of heavy elements and quantum materials. As our understanding of superatoms continues to expand, future research may unveil even more exotic molecular systems, establishing a basis for next-generation materials.