On the western shore of China’s Pearl River estuary in Zhuhai, scientists are harnessing renewable energy and seawater to mitigate the carbon emissions from the petrochemical sector by producing ‘green’ hydrogen. Researchers from Beijing University of Chemical Technology (BUCT) and the China National Offshore Oil Corporation (CNOOC) are setting up a pilot facility to conduct direct electrolysis of seawater into oxygen and hydrogen.
This technological leap poses considerable scientific challenges due to the corrosive and chemically complex properties of seawater. Additionally, renewable energy sources such as wind and solar operate sporadically, highlighted by BUCT’s Xiaoming Sun. In a Nature publication from March, Sun’s team introduced a corrosion-resistant catalyst that acts as an electrode within a seawater electrolysis system. This is vital, as inconsistent operations can trigger chemical processes that impair electrode performance.
The driving force behind this initiative is the vision of a decarbonized future where green electricity and hydrogen dominate the energy landscape, as noted by Julian Gregory from the University of Exeter. However, due to the unpredictable nature of renewable energy, it is essential to create excess capacity to guarantee a stable supply. Gregory points out that this surplus electricity can economically be converted into green hydrogen through electrolysis, as large-scale electricity storage remains expensive.
A provocative issue arises: Is direct seawater electrolysis merely ‘a solution in search of a problem?’ This technology stands in competition with techniques that involve desalination-enhanced electrolysis. Despite the doubts, researchers remain committed to its promise, as evidenced by the Zhuhai pilot project and endeavors like the industrial-scale research initiative in Qingdao, China.
In the quest to advance amidst escalating global carbon emissions, such technologies are under pressure to prove their effectiveness. Research must progress swiftly to establish direct seawater electrolysis as viable in economically advantageous sectors. Ironically, mitigating emissions in the oil and chemical industries may emerge as a feasible niche for the success of direct seawater electrolysis.
Within the UK, a team from the University of Glasgow may have resolved some obstacles, as demonstrated in trials using Evian mineral water. While chemical compositions differ between Evian and seawater, both face similar challenges in water splitting.
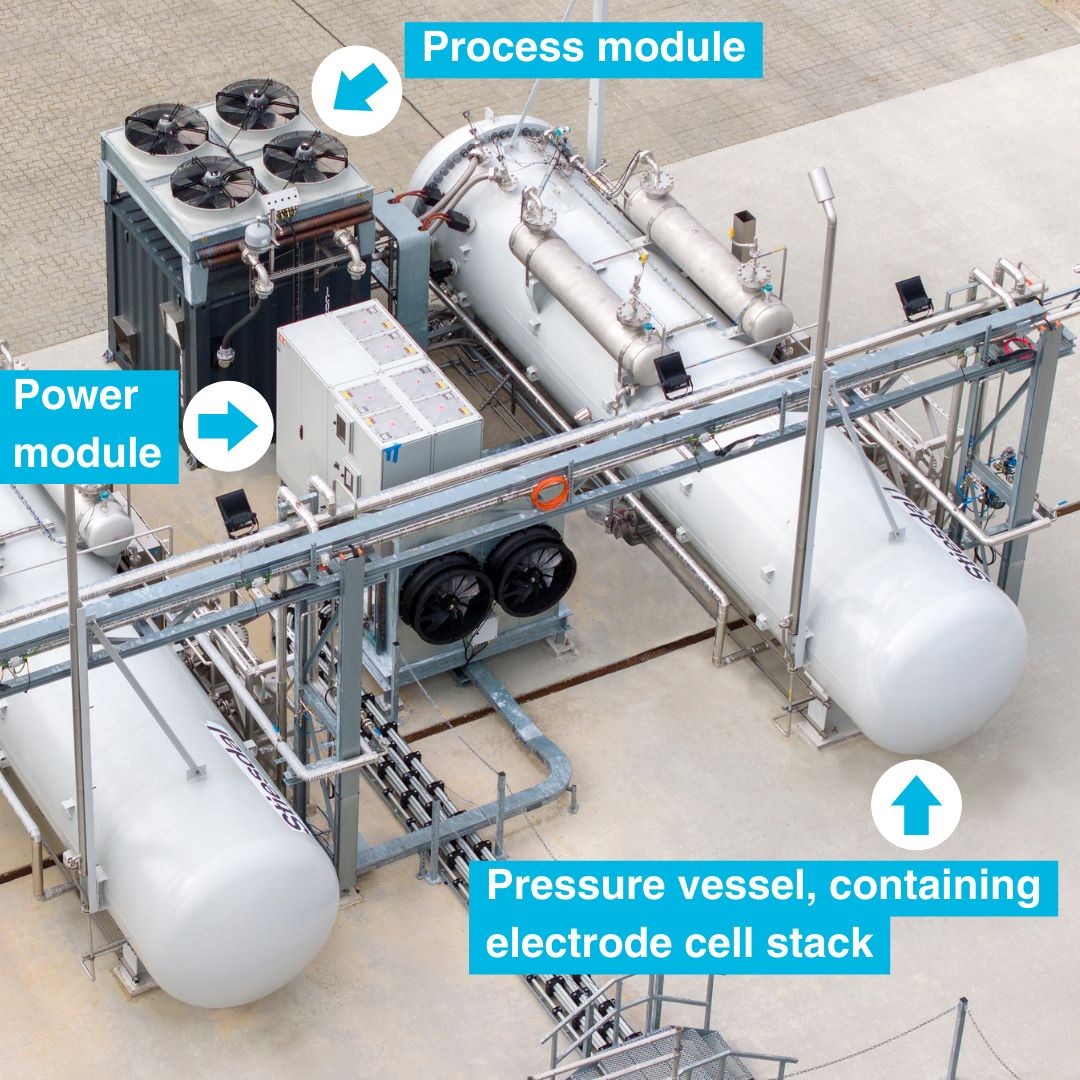
Electrolysis breaks down water into oxygen and hydrogen utilizing cells where electrons exit via the anode and return through the cathode. This process requires resilient electrocatalysts to facilitate reactions and reduce energy consumption.
Mark Symes and his colleagues at the University of Glasgow investigated proton-exchange membrane (PEM) cells, which are expected to optimally integrate with renewable energy sources. Unlike alkaline cells, PEM cells offer swift adaptability to power variations and provide a compact design, as described by energy advisor Titi Oliyide.
Even slight ion concentrations in Evian lead to quick malfunctions in PEM cells due to ion interference within the membrane, disrupting the electrolysis process. Given PEM’s sensitivity essential for leveraging the abundant seawater resources needed for green hydrogen, desalination—even at minimal cost increases—poses significant challenges since pure water suitable for PEM cells would raise expenses. Thus, introducing untreated seawater into electrolysers could be revolutionary, though it faces conventional difficulties.
Without electrolysis, reducing seawater still leads to metal corrosion and induces hydrogen embrittlement. Moreover, electrolysis can trigger fluctuations in seawater’s acidity and alkalinity, causing troublesome precipitates to form at the cathodes and disrupting processes by generating chlorine instead of oxygen at the anodes.
The Glasgow team addressed PEM issues with an innovative cell design employing sodium-permeable membranes and carbon cane cathodes; these solutions carry protons and electrons separately, releasing them through a catalyst like platinum. The separation using polyoxometalate solutions overcomes the blocking issues of PEM, enabling continuous current flow.
A spin-off, Clyde Hydrogen Systems, is pursuing commercial feasibility, concentrating on modular systems to mitigate corrosion effects. The environmental impact of current PEM membranes—characterized as ‘forever chemicals’—drives progress towards membraneless electrolysis systems.
Among these advancements is Shyp’s membraneless seawater electrolysis technology, producing valuable hydroxide byproducts to help offset costs of green hydrogen, moving towards demonstrators for scaling. Similarly, Sun’s research group is investigating anion-exchange membranes (AEM), which utilize polymeric electrolytes, although adoption has been slow due to degradation concerns.
Electrocatalyst innovation focuses on enhancing performance and longevity, with Sun’s colleagues developing corrosion-resistant NiCoP–Cr₂O₃ electrocatalysts that withstand operational cycles and hinder ion deposition at the cathodes.
Established technologies for desalination-enhanced electrolysis, such as those at NEOM in Saudi Arabia, surpass pilot projects in scale but still leave direct seawater electrolysis suited for specialized applications like offshore platforms that lack space for desalination or require minimal maintenance.
Initiatives like Sinopec’s Qingdao facility, powered by dedicated solar energy, aim to incorporate green hydrogen into refining operations or as fuel for vehicles. However, this application is argued to be economically less favorable compared to electric engines or heating, according to Gernot Wagner from Columbia Business School.