### Introduction to CRISPR
CRISPR represents a groundbreaking mechanism that is reshaping lives and generating significant interest in the healthcare sector. CRISPR, an acronym for “Clustered Regularly Interspaced Short Palindromic Repeats,” exists in prokaryotes—tiny, unicellular organisms lacking organelles. These sequences are embedded in the genomes of prokaryotes, which accumulate a cell’s DNA in clustered formations.
As a gene modification instrument, CRISPR is utilized to alter precise segments of DNA with the aim of addressing serious medical conditions. Its notable utility lies in its ability to protect the organism from viral attacks by integrating the foreign DNA into its own genome, thus facilitating recognition and destruction of the virus upon subsequent exposures. Remarkably, in contrast to alternative gene editing techniques, CRISPR is significantly more accurate and simpler to program, streamlining the sequence redesign process. While other gene-modifying tools generally depend on a single protein, CRISPR employs RNA-guided targeting paired with the Cas9 enzyme.
—
### How CRISPR Works
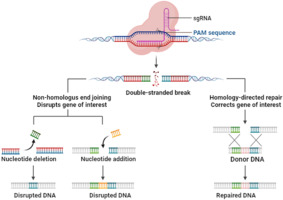
CRISPR operates by utilizing a natural defense system observed in bacteria that empowers them to detect and eliminate viral threats. When a virus infiltrates a bacterial cell (a prokaryote), the bacterium preserves a portion of the invader’s DNA within its genome as a genetic “archive.” This enables the bacterium to identify and respond more adeptly to subsequent infections.
In gene editing, this framework is modified utilizing two essential components: the Cas9 enzyme, which acts as molecular scissors to cleave DNA, and guide RNA, which steers Cas9 to the precise genetic area that requires modification. After the targeted DNA is severed, the cell’s inherent repair mechanisms take charge, allowing researchers to implement changes to the genetic structure.
Contrary to previous tools that depended on difficult-to-reprogram proteins, CRISPR’s RNA-guided system is much more adaptable, simpler to design, and highly accurate. This ease of use and precision has enabled the application of CRISPR in fields such as healthcare, agriculture, manufacturing, and microbiology—like engineering microbes to enhance product yields. Nevertheless, as this article discusses, as capabilities broaden, so do ethical dilemmas, especially concerning germline editing and genetic improvement.
—
### World’s First Patient of a Personalized CRISPR Treatment
In February 2025, the first personalized CRISPR treatment was carried out for an infant named KJ to address a deficiency in Carbamoyl Phosphate Synthetase 1 (CPS1)—an enzyme crucial for converting ammonia (produced during protein degradation) into urea. A research team led by Dr. Rebecca Ahrens-Nicklas and Dr. Kiran Musunuru at the Children’s Hospital of Philadelphia created this therapy after extensive research in gene editing and collaboration with fellow clinicians.
Their focus was on conditions impacting the urea cycle, leading to a dangerous accumulation of ammonia that can damage organs such as the brain and liver. They specifically tailored the treatment to KJ’s type of CPS1 deficiency based on preclinical research involving similar variants.
Up until now, the only CRISPR therapies sanctioned by the U.S. FDA have addressed more prevalent diseases like sickle cell disease and beta thalassemia, which impact tens or hundreds of thousands of individuals. In KJ’s scenario, his treatment was developed within six months post-birth, specifically targeting his unique CPS1 variant. The team devised a base editing therapy that was delivered via lipid nanoparticles to his liver to rectify the defective enzyme.
The February treatment constituted the first of three doses; KJ received the subsequent two in March and April 2025. As of his last dose, he has not suffered serious side effects, demonstrates improved tolerance to dietary protein, and requires reduced medication to manage ammonia levels. Although he will necessitate continuous monitoring, Ahrens-Nicklas indicates that the outcomes thus far are encouraging.
—
### Ethical Considerations of CRISPR
Similar to any pioneering technology, CRISPR prompts intricate ethical dilemmas. While its primary aim is to edit somatic cells for therapeutic purposes, it can also be applied to gametes, entering the contentious domain of germline editing. Modifying DNA that can be passed on to future generations is frequently perceived as unethical—particularly if done for enhancement rather than for medical treatment.
In light of these issues, scientists have temporarily suspended germline editing until its ethical and societal ramifications are fully comprehended. The unpredictable nature of genetic modifications, along with their permanence across generations, poses critical inquiries regarding where to establish boundaries. What originated as a creative concept has evolved into a formidable reality, igniting discussions about who holds the authority to determine its limitations.
The substantial costs and sophisticated infrastructure requirements of CRISPR render it more attainable in affluent, developed countries—especially in the West. As treatments continue to be costly, access becomes restricted to the wealthy, exacerbating socioeconomic disparities. The potential to utilize CRISPR to create so-called “