Overview of CRISPR
CRISPR represents a groundbreaking innovation that is reshaping lives and generating significant advancements in healthcare. CRISPR, which denotes “Clustered Regularly Interspaced Short Palindromic Repeats,” is present in prokaryotes—microscopic, single-celled entities lacking organelles. These sequences exist within the genomes of prokaryotes, which contain a cell’s DNA organized in bundles.
As a gene modification system, CRISPR is employed to alter specific sections of DNA to combat critical diseases. It is notably effective in protecting the body from viral assaults by integrating the foreign DNA into its own genome, enabling it to identify and eliminate the virus in future instances. What stands out is that, in comparison to alternative gene editing techniques, CRISPR is significantly more accurate and simpler to program, facilitating a more straightforward sequence alteration process. While traditional gene-editing instruments generally utilize a single protein, CRISPR employs RNA-guided targeting alongside the Cas9 enzyme.
The Mechanism of CRISPR
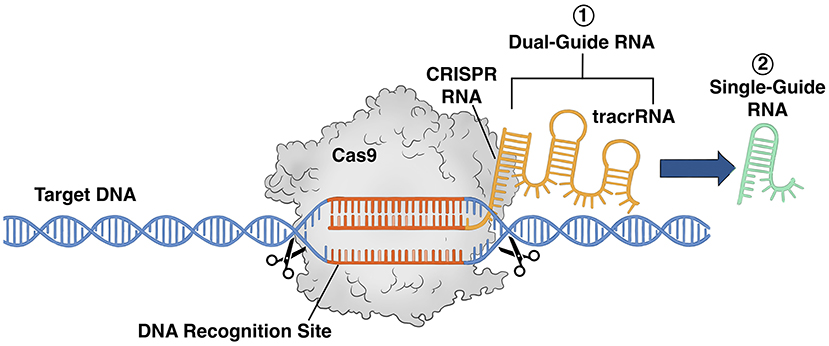
CRISPR operates by leveraging a natural defense strategy found in bacteria that enables them to detect and eradicate viral threats. When a virus infiltrates a bacterial cell (a prokaryote), the bacterium retains a portion of the invader’s DNA within its own genome, functioning as a genetic “memory.” This allows the bacterium to identify and react more proficiently to subsequent infections.
In gene editing, this mechanism is modified with two essential elements: the Cas9 enzyme, which acts like molecular scissors to sever DNA, and guide RNA, which directs Cas9 to the particular genetic sequence needing modification. Once the desired DNA is severed, the cell’s intrinsic repair processes assume control, allowing researchers to implement alterations to the genetic makeup.
Distinct from older tools that depended on challenging-to-reprogram proteins, CRISPR’s RNA-guided method is more adaptable, simpler to design, and extremely precise. This straightforwardness and accuracy have permitted CRISPR’s application in medicine, agriculture, manufacturing, and microbiology—such as modifying microbes to boost product outputs. However, as this article examines, with broadened capabilities come rising ethical dilemmas, particularly concerning germline editing and genetic enhancement.
First Patient Undergoing Personalized CRISPR Therapy
In February 2025, the inaugural personalized CRISPR therapy was administered to an infant named KJ to address a deficiency in Carbamoyl Phosphate Synthetase 1 (CPS1)—an enzyme essential for converting ammonia (produced during protein degradation) into urea. A team helmed by Dr. Rebecca Ahrens-Nicklas and Dr. Kiran Musunuru at the Children’s Hospital of Philadelphia created this therapy after extensive research in gene editing and collaboration with various medical professionals.
Their efforts centered on conditions impacting the urea cycle, leading to harmful ammonia accumulation, which can damage organs such as the brain and liver. They customized the treatment specifically for KJ’s particular variant of CPS1 deficiency utilizing prior research on analogous cases.
So far, the only CRISPR therapies sanctioned by the U.S. FDA have been for more prevalent disorders like sickle cell disease and beta thalassemia, which affect thousands of individuals. In the case of KJ, his therapy was tailored within six months of his delivery, focusing on his unique CPS1 variant. The team formulated a base editing treatment administered via lipid nanoparticles to his liver to rectify the defective enzyme.
The February session marked the first of three doses; KJ received the subsequent two in March and April 2025. As of his final dosage, he has shown no significant adverse reactions, demonstrates improved tolerance to dietary protein, and requires less medication to regulate ammonia levels. Although continuous monitoring will be necessary, Ahrens-Nicklas indicates that the outcomes thus far are encouraging.
Ethical Implications of CRISPR
As with any transformative technology, CRISPR poses intricate ethical issues. While its main goal is to modify somatic cells for disease treatment, it can also be applied to gametes, entering the contentious area of germline editing. Modifying DNA that will be passed down to future generations is frequently perceived as unethical—particularly when aimed at enhancing traits rather than addressing health conditions.
In light of these apprehensions, researchers have temporarily halted germline editing until its ethical and