Iron supplements frequently remedy blood issues but can cause gut discomfort. A newly developed lab formulation strives to achieve both outcomes effectively, having recently completed preliminary trials in mice as reported in a peer-reviewed journal by the American Chemical Society.
Researchers from India have designed a synbiotic iron delivery mechanism that combines an iron complex with a probiotic and its ideal nutrients within a mucoadhesive hydrogel. In anemic mice, a two-week treatment period improved hemoglobin levels, reduced inflammatory markers in the colon, and helped restore gut microbial balance. The findings, published in ACS Applied Materials & Interfaces, indicate a potential strategy to address iron deficiency anemia while safeguarding the intestines instead of aggravating them.
If you’ve ever unwrapped a typical iron tablet, you’re familiar with its appearance and odor: a chalky disk that can feel heavy in the stomach. This innovative material functions more like a gentle, protective raft. Envision a transparent gel bead, roughly the size of a seed pearl, that adheres to the intestinal wall and gradually releases its contents as it moves through the moist channels of the gut.
What Sets The New Formulation Apart
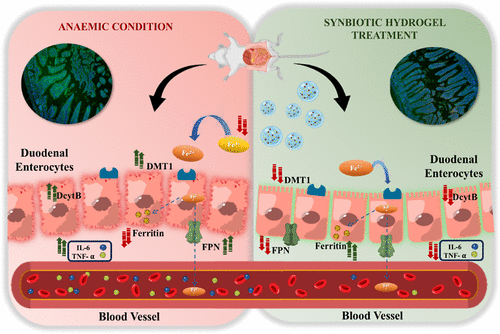
The researchers integrated three components into one: an iron dextran complex for restoring blood iron levels, live Lactobacillus rhamnosus as a probiotic, and soluble dietary fiber from millet to nourish this microorganism. All three components were encapsulated in a thiolated hyaluronic acid hydrogel intended to adhere to mucus and regulate release. In cellular experiments, the formulation proved biocompatible. In animal studies, it not only elevated hemoglobin but also shifted molecular indicators in ways that aligned with improved iron metabolism and lower inflammation.
The researchers monitored expressions linked to iron transport, such as DMT1 and DcytB, and noted normalization following treatment. Ferritin protein levels increased, signaling restored reserves. Colon tissue exhibited decreased TNF alpha and IL 6, confirmed by immunofluorescence and Western blot analyses. Fecal iron concentrations matched those of non-anemic controls, indicating that more iron was absorbed rather than left to irritate the lumen. Microbiome profiles also moved back toward baseline. These findings are based on mouse data instead of human results, yet they address the fundamental dilemma clinicians encounter with traditional iron salts: effective systemic replenishment at the expense of local gut disruption.
“By enhancing biomaterial-based iron delivery, this study presents a groundbreaking method to combat anemia, significantly contributing to improved nutrition and long-term public health.”
This assertion will require human trials to be substantiated. Nonetheless, the underlying engineering rationale is robust. Encapsulation can protect delicate microbes and control the release of both iron and prebiotic components. Mucoadhesion helps retain the delivery in the vicinity of transporters. Combining a probiotic with its preferred fiber can shift the local ecosystem away from inflammatory conditions that free iron might encourage.
Importance For Patients And Public Health
Iron deficiency anemia persists as a widespread issue across various demographics and environments. Oral iron is economical and scalable, but side effects often lead to poor compliance. A formulation that enhances absorption while minimizing gut irritation could significantly improve real-world effectiveness. It might also lessen the requirement for higher doses, which typically exacerbate gastrointestinal issues. From a systems perspective, the components are familiar, the mechanism is delivery, and the outcome is a better equilibrium between effectiveness and tolerability.
However, there are challenges. Mouse intestines differ from human intestines. Regulatory pathways for live biotherapeutics can be intricate. Stability during production and storage requires demonstration. Dosing regimens and interactions with antibiotics or existing probiotics require investigation. Cost considerations will also be crucial. Nevertheless, this approach combines known elements into a coherent design, which often leads to quicker results than starting from the ground up.
“The results indicated progress toward creating an integrated oral iron supplementation medium with enhanced bioavailability and minimized gut inflammation.”
Future steps should involve randomized, controlled human trials assessing not only hemoglobin restoration and ferritin levels but also stool iron, calprotectin, patient-reported gastrointestinal experiences, and microbiome changes over time. Direct comparisons with standard ferrous sulfate would clarify the treatment’s value. If the results are consistent, this could represent a minor but significant enhancement to a therapy used by millions.
For the time being, the study delivers a clear message: delivery details are crucial. By refining the microenvironment at the mucosal layer and combining iron with supportive elements instead of allowing it to react unopposed, it may be possible to enrich blood while protecting the gut simultaneously.