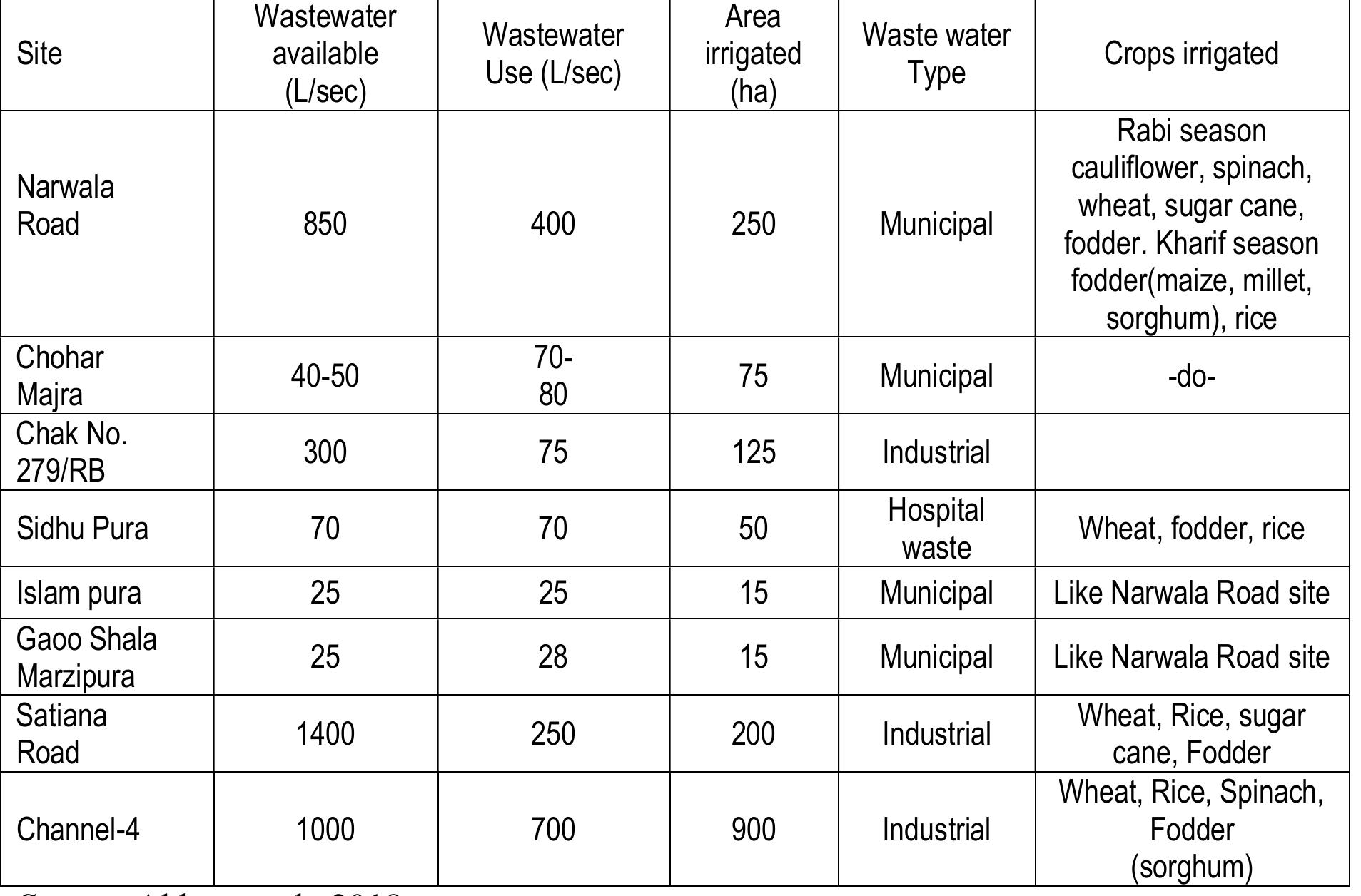
Imagine this: 8,000 years in the past, your forebears are discovering how to cultivate wheat and barley, tending to goats, and living closely with many neighbors for the first time in human history. Then, catastrophe strikes when someone becomes severely ill, experiencing days of vomiting. Within weeks, the entire community falls ill. Time and again.
Scientists now believe this harrowing event is precisely what caused a fortunate mutation to proliferate among early farming populations. Some individuals possess a flawed gene that grants them complete immunity to Norovirus, the pathogen responsible for winter vomiting disease. It appears this genetic barrier emerged not by coincidence, but because agricultural practices rendered us susceptible in ways our hunter-gatherer predecessors were not.
“We aimed to track the historical propagation of the gene variant,” states Hugo Zeberg, a geneticist from Karolinska Institutet and the Max Planck Institute for Evolutionary Anthropology. His research group examined DNA from 4,343 ancient individuals over the past 10,000 years, effectively rewinding evolution to observe natural selection in progress.
When Close Living Conditions Became Lethal
The protective mutation pertains to a gene named FUT2, which typically aids in producing an enzyme within the intestinal lining. This enzyme embellishes gut cells with sugar molecules, which Norovirus attaches to in order to infiltrate. Individuals with the defective variant are unable to utilize the enzyme properly. No sugar molecules mean no entry point for the virus. It simply slips off harmlessly.
The mutation first emerged in Europe around 6,000 BCE, brought by early farmers venturing from what is now Turkey. However, the intriguing part is that the gene didn’t merely seep through the population. Between 8,500 and 5,000 years ago, it surged in prevalence.
“Our findings indicate that this kind of disease environment amplified the frequency of the gene variant as it offers protection against winter vomiting disease and gives an advantage to those carrying it by not falling ill.”
The timing is intentional. Early farming communities established permanent settlements, frequently characterized by inadequate sanitation and much closer living conditions compared to nomadic societies. Norovirus propagates through tainted food and water, thriving under precisely these circumstances. One infected individual in a small farming community could initiate an outbreak that repeatedly swept through, since immunity to Norovirus is notoriously brief. It is possible to contract it multiple times within a single season.
Current Evidence from Miniature Intestinal Models
To validate their evolutionary investigation, Zeberg’s group utilized modern biobank data from 700,000 individuals. Those possessing two copies of the protective variant, one from each parent, almost never reported episodes of winter vomiting disease. They then advanced their research by cultivating human intestinal organoids, effectively miniature guts derived from biopsy samples, and exposed them to Norovirus.
The outcomes were striking. Individuals with double copies of the mutation demonstrated complete protection. The virus could not establish a foothold.
“Understanding why certain mutations occur and are selected helps us gain insight into how they impact our health today,” notes Johan Nordgren, the lead author of the study and a docent at Linkoping University.
About twenty percent of Swedes carry two copies of this protective gene, rendering them essentially immune to Norovirus. Yet evolution seldom provides benefits without costs. The same mutation that offers protection against vomiting sickness seems to increase the risk of stomach ulcers and gallstones. Modern biobanks reveal a higher occurrence of these conditions in individuals with the variant.
“These are usually associated with stress and a high-fat diet, which was likely less prevalent during the neolithic era.”
In essence, the trade-off was sensible 8,000 years ago. Evading repeated instances of debilitating illness outweighed the slightly elevated risk of issues that may never arise in a low-fat, low-stress agricultural lifestyle. Today, with our sedentary occupations and rich diets, the calculations have changed.
For Zeberg, the clinical consequences are secondary to the evolutionary narrative. Prehistoric DNA, according to him, serves as a time machine. “It permits us to replay evolution and observe how genetic mutations can be connected to events in the human environment.” The study, published in Molecular Biology and Evolution, provides a unique glimpse into how a singular lifestyle shift, the transition to agriculture, left a lasting impact on our genomes.
And the next time Norovirus ravages your workplace or your child’s school, think for a moment about those early farmers. Their suffering became our legacy, at least for some of us.
[Molecular Biology and Evolution: 10.1093/molbev/msaf243](https://doi.org/10.1093/molbev/msaf243)
There’s no paywall here
If our reporting has informed or inspired you, please consider making a donation. Every contribution, regardless of size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible