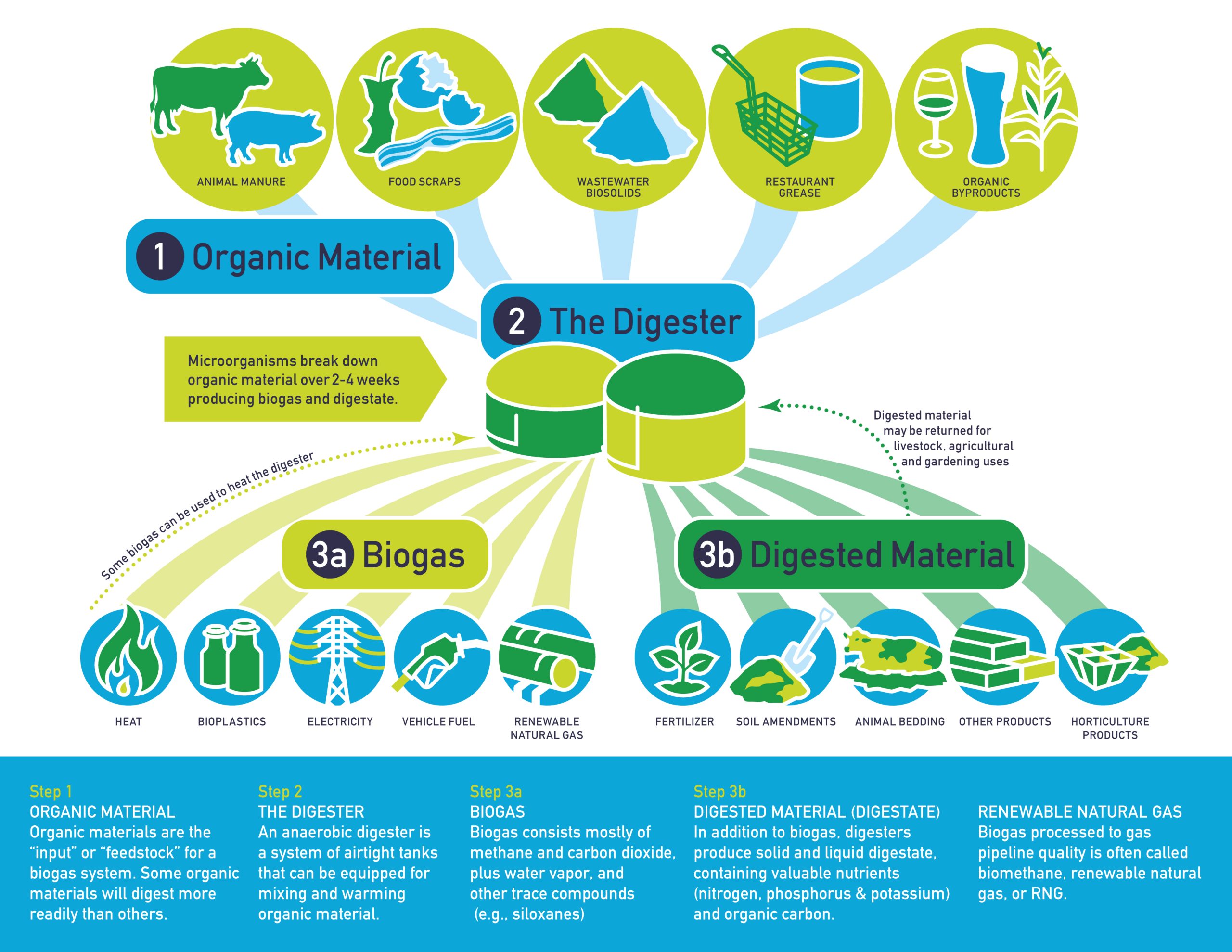
### A Major Advancement in Carbon Capture: Converting CO₂ into Methane using Renewable Energy
As global climate issues intensify, the need to employ cutting-edge technologies for capturing and repurposing greenhouse gases has emerged as a vital scientific focus. Recent breakthroughs by chemists at The Ohio State University have introduced a revolutionary method to tackle one of the most urgent challenges facing humanity: effectively managing carbon dioxide (CO₂) emissions. By utilizing a unique nickel-based catalyst, these researchers have created a technique that not only captures CO₂ but also converts it directly into methane—a high-energy fuel—through the use of renewable electricity. This innovation could revolutionize carbon capture and utilization, potentially transforming climate mitigation strategies.
#### The Challenge of CO₂ Emissions
CO₂ is the key greenhouse gas responsible for escalating global temperatures, owing to its high levels in the atmosphere. It is released during the combustion of fossil fuels in power generation, industrial operations, transportation, and other energy-heavy activities. While current carbon capture, utilization, and storage (CCUS) technologies attempt to confine CO₂ and turn it into commercially viable products, these systems frequently prove to be energy-intensive and economically difficult to expand. Existing methods generally consist of several energy-demanding phases: capturing CO₂, reclaiming it, and then processing it into value-added materials. Such methods have hindered broader implementation in various industries.
#### The Introduction of the Nickel-Based Catalyst
The innovative study, conducted by Tomaz Neves-Garcia, a postdoctoral fellow at The Ohio State University, presents a more energy-efficient alternative. By merging the processes of carbon capture and conversion into a unified step, the research team successfully transformed CO₂ directly into methane, a high-energy fuel. Central to this process is a cost-effective nickel-based catalyst. When nickel atoms were arranged on an electrified surface, they allowed the immobilized CO₂—stored in carbamate form—to convert into methane through electrochemical reactions.
“What makes this so fascinating is that others capture, recover, and then convert carbon dioxide in separate steps,” noted Neves-Garcia. “We conserve energy by executing these steps at once.”
This single-step method eliminates the requirement for energy-intensive intermediate stages, significantly lowering the total energy needed to process captured CO₂. Achieving such a high-efficiency conversion represents a remarkable advancement in CCUS technology, as it points to new possibilities for more sustainable and cost-effective industrial practices.
#### The Vision of a Closed Carbon Cycle
The overarching objective of this research is to progress toward a closed carbon cycle where emitted CO₂ can be perpetually recycled into fuels or other valuable substances. Methane, for example, shows considerable potential: when utilized as a fuel, it releases energy while emitting CO₂, which can again be captured and converted back into methane using renewable electricity. This could establish a self-sustaining energy production cycle that reduces net CO₂ emissions and helps combat global warming.
“Transforming CO₂ into a fuel with renewable electricity has the potential to close the carbon loop,” remarked Neves-Garcia. “If this can be accomplished in a sustainable and scalable manner, it would mark a significant achievement in the fight against climate change.”
#### Electrochemistry: A Transformative Factor for CO₂ Conversion
Previously, efforts to repurpose captured CO₂ into useful materials have predominantly concentrated on producing carbon monoxide (CO), a simpler but less adaptable molecule than methane. This research is the first to showcase the practicality of employing electrochemistry to convert carbamate directly into methane, setting a new benchmark for creating higher-energy molecules.
“Methane can be an exceptionally valuable product,” added Neves-Garcia. “But most crucially, this opens the door to develop additional methods for converting captured CO₂ into a variety of products.”
#### Implications for Climate Mitigation Strategies
These discoveries carry broader ramifications for formulating responses to climate change. By enhancing the efficacy of carbon capture and conversion, the research reshapes how the carbon cycle is handled. This technique could effortlessly integrate with renewable energy sources, such as wind and solar, to drive electrochemical processes, offering a cleaner, more sustainable approach to energy production and greenhouse gas management.
Neves-Garcia highlighted the necessity of reducing energy expenses for feasible long-term solutions: “We should aim to minimize energy expenditure for carbon capture and conversion.” This level of efficiency is vital for CCUS systems to achieve widespread acceptance across various sectors, including manufacturing and power generation.
#### Collaborative Efforts and Future Perspectives
The study, recently featured in the *Journal of the American Chemical Society*, involved contributions from a diverse range of scholars, including experts from The Ohio State University, the University of Sao Paulo, and Case Western Reserve University, alongside Yale University and the Southern University of Science and Technology. Such collaborative endeavors emphasize the interdisciplinary nature of tackling global climate issues.
Looking forward, the team is eager to investigate alternative methods for sustainable carbon utilization. The nickel-based catalyst could serve as inspiration for further innovations in generating other chemical fuels or materials from captured CO₂.