**A Green Revolution: Scientists Develop Carbon-Negative Cement Production Technique**
Cement production, historically recognized as one of the most carbon-heavy industries, stands on the verge of a transformative change thanks to a novel electrochemical technique created by researchers at Lawrence Livermore National Laboratory (LLNL) and Northwestern University. This groundbreaking approach, capable of facilitating gigaton-scale reductions in global carbon emissions, has the potential to electrify and decarbonize the cement industry while easily fitting into its current infrastructure.
### The Cement Sector’s Ecological Dilemma
As a crucial material in the construction sector, cement is frequently overlooked. However, its manufacturing accounts for almost 8% of worldwide greenhouse gas emissions, making it the second-largest industrial emitter, following the energy sector. A significant portion of these emissions arises from the calcination of limestone—a process that consumes considerable energy and emits notable amounts of carbon dioxide (CO₂).
Current methods aimed at reducing the carbon footprint of cement generally involve adding industrial byproducts like coal fly ash and blast furnace slag into concrete mixtures. Unfortunately, these materials can only replace a limited part of cement’s ingredients, which restricts their effectiveness. Moreover, they face supply and performance limitations, keeping the industry reliant on conventional, carbon-heavy methods.
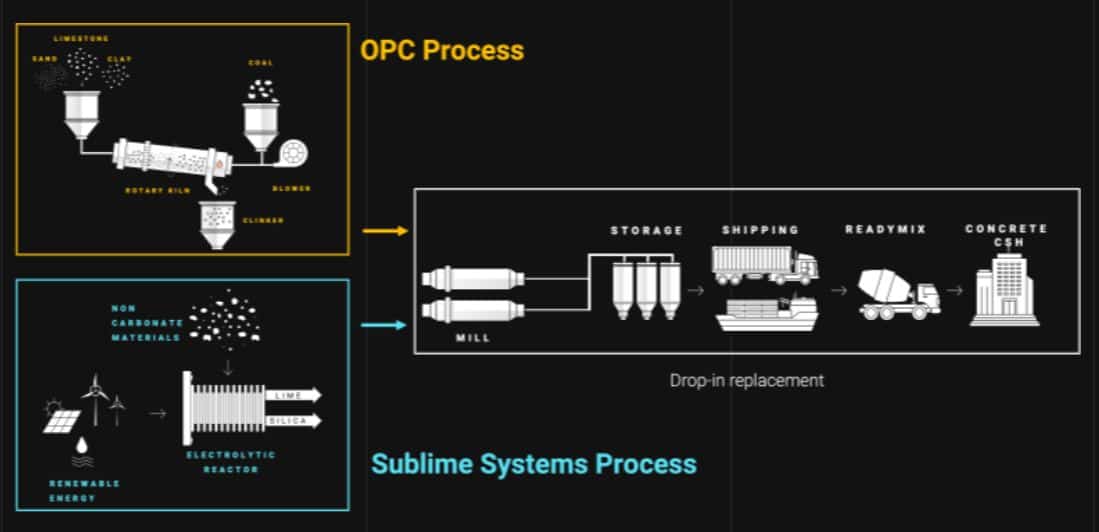
### A Revolutionary Electrochemical Approach
This recent advancement disrupts established practices by entirely eliminating the need for limestone calcination. The LLNL and Northwestern University team has created a distinctive electrochemical process that accelerates the natural weathering of calcium silicates derived from basalt rock, recycled concrete, and industrial waste. Importantly, this method utilizes CO₂ to produce carbon-negative calcium carbonate, the essential component for cement manufacturing.
“At the core of our strategy is an electrochemical cell that captures carbon dioxide and chemically converts it into calcium carbonate,” states Wenxin Zhang, a principal researcher at LLNL. The procedure initiates by establishing a pH gradient within the electrochemical cell, which extracts calcium ions from calcium silicate sources. These ions are subsequently combined with CO₂—either from the atmosphere or from post-industrial carbon capture systems—to yield calcium carbonate and amorphous silica.
### Seamless Integration with Current Systems
One of the most advantageous features of this innovation is its alignment with existing cement production infrastructure. Since calcium carbonate continues to be the main raw material for cement production, this method can be adopted within current facilities with minimal modifications. “It’s very feasible,” Zhang points out, “as our approach doesn’t necessitate major changes to established manufacturing systems.”
Moreover, the amorphous silica generated during the process acts as a supplementary cementitious material. Not only does it enhance the durability and strength of the resulting concrete, but it also contributes to a greater carbon-negative effect when mixed with cement.
### Circular and Renewable: An Ambient Temperature Process
Another impressive characteristic of this method is its energy efficiency and circularity. Unlike traditional cement manufacturing, which typically requires temperatures exceeding 1,400°C, the electrochemical technique functions at room temperature. This reduces reliance on fossil fuels for heating and facilitates the operation of the system using renewable energy sources like wind or solar—regardless of their intermittency.
In addition to generating cement precursors, the process produces valuable byproducts, such as green hydrogen and oxygen. Green hydrogen, a clean energy carrier, can be utilized within the cement plant as well as in other industrial settings, potentially substituting fossil fuels as a heat source. Meanwhile, oxygen could facilitate oxy-fuel combustion, enhancing fuel efficiency and lowering emissions from calcium carbonate breakdown. Furthermore, captured CO₂ might be transformed into calcium bicarbonate to mitigate ocean acidification, creating new environmental solutions.
### Professional Validation and Economic Promise
John Provis, a distinguished cement systems authority from the Paul Scherrer Institute, acknowledges the innovation’s promise: “The circularity is developed in some very fascinating ways. The researchers have clearly considered process integration and synergies that can bolster the environmental benefits of their approach.” While he highlights that the method necessitates a decarbonized electricity supply to maximize effectiveness, he recognizes the lucrative potential of the byproducts it produces.
From an economic standpoint, this technology could signify a substantial advancement. By leveraging readily accessible raw materials like basalt, recycled waste, and CO₂, production costs could decrease. In addition, the reduced retrofitting needs imply lower financial hurdles for cement producers. The commercialization of co-products such as green hydrogen and carbon credits could also create new revenue streams, offering a compelling financial incentive for firms to embrace this innovative process.
### Expanding the Future of Cement
Although the laboratory outcomes have been revolutionary, the research team’s next phase is to investigate the scalability of their solution. They intend to perform location-specific studies, considering regional energy frameworks, availability of raw materials, and economic factors. These analyses will yield practical strategies for scaling the technology and adapting it for industrial use.
Ultimately, this innovation holds the promise of transforming the cement sector and