The initial large-scale synthesis of hexagonal diamond signifies a significant advancement for carbon allotropes, providing researchers with a chance to thoroughly characterize this distinctive material.
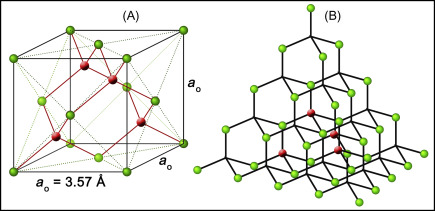
Diamond ranks among the toughest materials recognized in nature, stemming from its structure where carbon atoms are covalently bonded in an ideal tetrahedral formation. Almost 60 years ago, scientists theorized a harder alternative known as hexagonal diamond, which features a hexagonal lattice instead of the cubic lattice found in traditional diamond.
Natural hexagonal diamond has been located on Earth and is believed to have formed during meteorite impacts when extremely high temperatures and pressures can swiftly convert graphite into this uncommon variant of diamond. Nonetheless, only tiny grains of this natural hexagonal diamond have ever been found, intermixed with cubic diamond and graphite. Techniques that mimic meteorite impact conditions in laboratory settings have frequently produced nanocrystalline forms of hexagonal diamond, with these samples often being impure, complicating the study of hexagonal diamond in a pure state.
‘Currently, we have created a millimeter-sized piece of [near] pure hexagonal diamond,’ states Ho-Kwang Mao at the Centre for High Pressure Science and Technology Advanced Research in China.
To achieve this, Mao and his team from the US and China subjected a single crystal of pure graphite to roughly 200,000 times atmospheric pressure using a diamond anvil cell. In situ x-ray diffraction conducted before and after the application of this pressure allowed researchers to witness the microscopic transformation of graphite into hexagonal diamond. While the sample remained under pressure, laser heating at 1400°C stabilized the phase, enabling the recovery and subsequent analysis of the near-pure sample.
‘This innovative two-step approach delivers the first conclusive proof of hexagonal diamond as a separate and recoverable bulk material,’ asserts Eiichi Nakamura, an inorganic chemist from the University of Tokyo who has previously studied carbon allotropes.
Through this methodology, the team produced crystals of near pure hexagonal diamond – comprising a few microcrystals of the more widely recognized cubic diamond. The crystals varied in size from 100μm to several millimeters, marking a significant achievement in creating a distinct and recoverable quantity of the material.
‘When you consider [the bonding arrangement of carbon atoms] in three dimensions, there are only two arrangements for stacking the layers,’ explains Mao. ‘There’s ABC packing in cubic diamond, and AB packing in hexagonal.’ High-resolution transmission electron microscopy validated that the sample exhibited AB stacking of buckled honeycomb layers, a structure characteristic of hexagonal diamond.
The scientists examined the structure more deeply using various spectroscopic techniques. ‘We discovered that one of the bonds between the layers is indeed shorter than the other three, helping to clarify why the structure is stronger [when compared to cubic diamond],’ notes Mao. Results also indicated that all bonds were sp^3 σ bonds, lacking any sp^2 π bonds that would indicate the presence of graphite.
The team assessed the material’s hardness with a 1mm diameter disc of hexagonal diamond, finding that its hardness was comparable to that of natural diamond, attributed to minor defects of cubic diamond. Future endeavors will likely aim at honing synthesis conditions, with Nakamura remarking that ‘this [synthetic] advancement signifies a pivotal moment in the exploration of carbon allotropes.’