A recent breakthrough in science may shed vital light on the mystery of how proteins first emerged on early Earth. Researchers have discovered that sulfur-containing intermediates could be essential for understanding how proteins assemble from basic molecules. This discovery bypasses the need for enzymes in the initial stages of protein synthesis, a process that has been deemed difficult to replicate.
In contemporary biology, protein synthesis is a meticulously controlled, enzyme-dependent procedure. The current method of ribosomal peptide synthesis involves the transfer of activated amino acids to transfer RNA (tRNA), which then aids in forming peptide bonds at ribosomes. However, this method assumes that proteins are required to produce enzymes, creating a paradox regarding the origins of the first proteins.
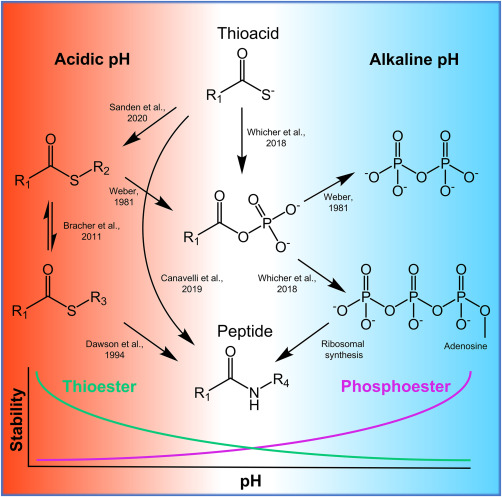
Significant strides have been made in addressing this paradox, especially concerning the aminoacylation reaction that traditionally necessitates enzyme catalysis. Earlier studies proposed several chemical activators, yet most were overly reactive, leading to undesired side reactions and instability in water-based environments.
Matthew Powner’s research group at University College London has suggested a more viable mechanism that features thioesters—functional groups commonly found in metabolic activities. Their experiments indicated that aminoacyl–thiols arising from prebiotic conditions can selectively bind to tRNA molecules, effectively facilitating the peptide synthesis pathway. This reaction accommodates a variety of amino acids while minimizing undesirable side reactions, echoing the dual function of ATP and synthetase enzymes in today’s biology.
With the support of hydrogen sulfide, the thioester mechanism evolves to create a thioacid, which can catalyze peptide bond formation without the involvement of enzymes. The study provides a proof-of-concept for enzyme-free peptide synthesis, initially focusing on creating a single peptide bond, with ambitions to expand this to synthesize larger polypeptides.
The origin of the thioester components is believed to stem from amino nitriles reacting with pantetheine, a molecule potentially common in the early Earth’s environment, as indicated by prior research from Powner and collaborator Saidul Islam at King’s College London.
Despite these advancements, uncertainties persist, particularly regarding how the genetic code accurately directed peptide sequence formation in such primitive conditions. The recognition of amino acids by tRNA units usually relies on specific nucleotide sequences, but this connectivity is absent without enzyme-driven mechanisms.
Islam emphasizes the sophistication of this research, highlighting the discovery of straightforward solutions through natural chemical reactivity in a domain often confronted with challenging problems. This study not only supports existing theories on prebiotic chemistry but also paves the way to align genetic sequences with peptide synthesis, ultimately unraveling the origins of life’s intricate molecules.