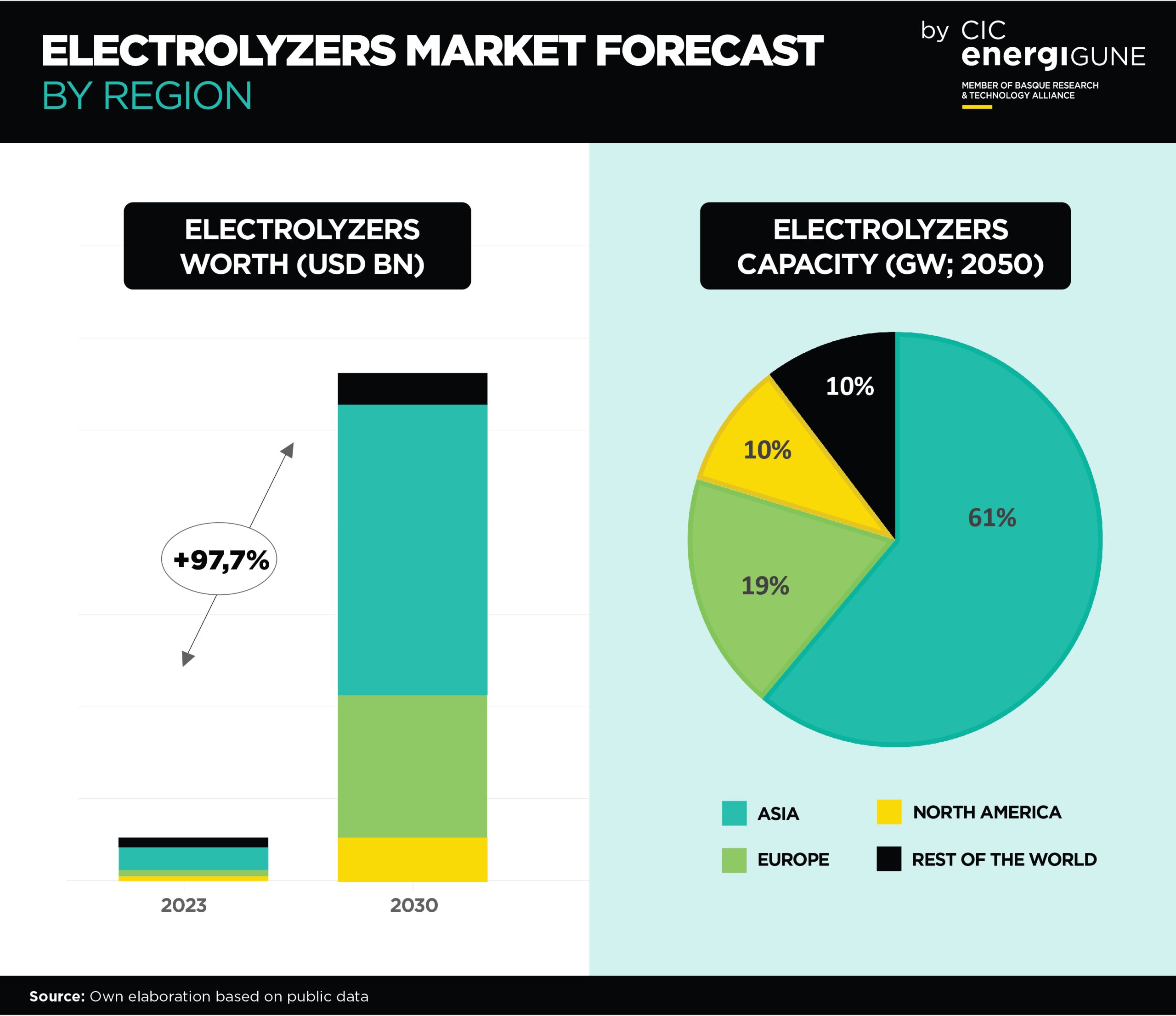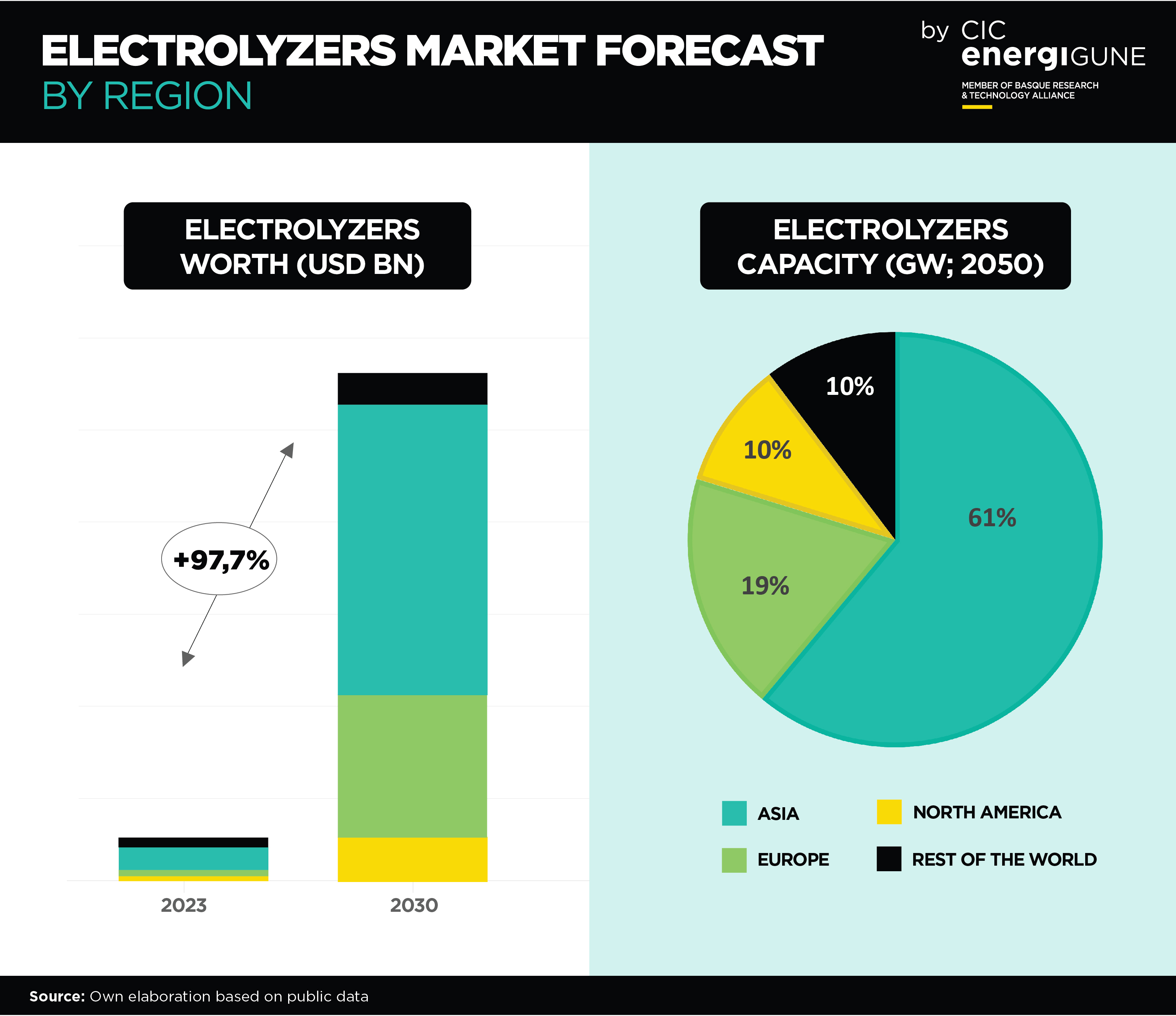
Advancements in Hydrogen Storage: A Significant Development with Solid Electrolytes
Hydrogen is recognized as a sustainable energy alternative, but its storage typically demands elevated pressure or heat. Scientists have been exploring hydrogen compounds to tackle storage issues but faced obstacles, including high operating temperatures and limited capacity. At the Institute of Science in Tokyo, researchers spearheaded by Naoki Matsui and Ryoji Kanno have created a cutting-edge solid electrolyte for effective hydrogen storage.
At present, two main approaches are available for solid-state hydrogen storage. The thermodynamic approach involves alloys that absorb hydrogen to generate compounds releasing hydrogen at high temperatures. Although certain metal hydrides, such as MgH₂ and LiH, offer substantial capacity, they necessitate approximately 300°C to liberate hydrogen. Another approach, suggested by Stanford’s Robert Huggins in 1985, consists of electrochemically inserting hydride ions through a liquid electrolyte. However, elevated temperatures also complicated this technique. Developments in solid-state electrolytes present a potential solution, guaranteeing stability and conductivity at reduced temperatures.
Matsui and Kanno employed superionic conductors to synthesize a compound comprising barium, calcium, and sodium ions (BaH₂–CaH₂–NaH), realizing high hydride ion conductivity. Their testing system integrated molybdenum current collectors and a reference configuration featuring TiH₂ and titanium electrodes. This study showcased the remarkable potential window of the solid electrolyte.
The researchers attribute the elevated hydride ion conductivity to the body-centered cubic (bcc) configuration of their electrolyte, which provides a clear pathway for ion passage. The polarizable cations within the structure assist in minimizing repulsion, further boosting conductivity.
To confirm hydrogen storage capabilities, they assembled a cell using magnesium hydride anode and LaHx cathode, achieving MgH₂’s theoretical capacity over five cycles at 90°C with a 0.12V potential.
Their findings represent a noteworthy advance, with Genki Kobayashi from RIKEN highlighting its landmark significance in electrochemical hydrogen storage. Matsui and Kanno intend to refine solid electrolytes with enhanced ionic conductivity and create devices with reduced operational temperatures and improved energy efficiency.