Photochemistry has surfaced as an excellent avenue for the alteration of oxetane rings by substituting the oxygen atom with nitrogen, carbon, or sulfur in a singular, effective maneuver. This groundbreaking technique opens the door to four-membered heterocycles like azetidines and thietanes, structures that have historically posed difficulties in synthesis but possess significant promise for medicinal uses. As noted by Karen de la Vega from the Institute of Chemical Research of Catalonia, this progress broadens the scope of skeletal editing beyond aromatic frameworks, presenting new prospects in drug development.
Oxetanes act as adaptable motifs in medicinal chemistry, frequently utilized as bioisosteres to replace carbonyls, thereby improving the stability of pharmaceutical compounds while preserving biological efficacy. The capacity to directly convert oxetanes into alternative four-membered rings paves the way for innovative drug creation, as observed by Jianwei Sun of the Hong Kong University of Science and Technology.
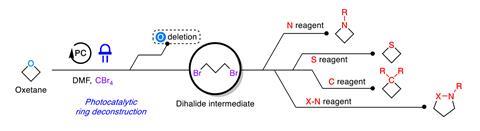
The groundbreaking atom-swapping technique offers an eco-friendly substitute to conventional methods, which typically require breaking down the rings into simpler elements—a laborious process laden with waste. Ming Joo Koh from the University of Singapore envisions a simplified, two-step approach that initiates with exposing oxetane to blue light alongside a ruthenium photocatalyst, resulting in an open dibromide intermediate. This intermediate can be effortlessly transformed into various strained rings through reactions with multiple nucleophiles.
This method not only improves the flexibility and applicability of oxetanes but also bypasses the prolonged and substrate-dependent traditional synthesis of saturated heterocycles. By enabling direct access to a variety of ring systems, it supports late-stage modifications to improve drug solubility and metabolic stability. Koh’s research on substituting oxygen with sulfur in a pharmaceutical candidate showcases the tangible advantages, as the resulting thietane derivative demonstrated significantly enhanced potency.
With this atom-swapping technique, chemists acquire a robust mechanism for producing drug analogues with potentially enhanced properties, greatly progressing the domain of medicinal chemistry.