**Title: Synthetic Hybrid Protein Derived from Insect Saliva Opens New Avenues in Anticoagulant Innovation**
A team of researchers, spearheaded by Richard Payne and Joshua Maxwell from the University of Sydney, has achieved a breakthrough in developing a synthetic protein that displays unrivaled binding affinity to thrombin, an enzyme pivotal in blood coagulation. By utilizing peptide fragments sourced from the saliva of a leech, a tsetse fly, and a rat flea, the researchers have crafted a novel hybrid molecule, XChimera, which holds considerable promise for anticoagulant advancement.
Cardiovascular ailments, significantly impacted by thrombotic incidents such as strokes and heart attacks, continue to be the leading causes of mortality globally. Present anticoagulant medications, though effective, carry risks of bleeding and demonstrate a limited therapeutic window, especially during surgical interventions or in the prevention of coronary artery disease. This scenario fosters the quest for safer and more refined alternatives.
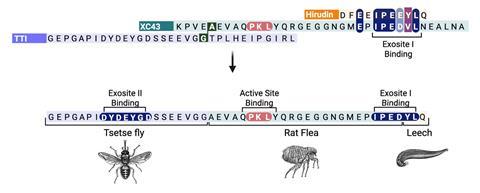
XChimera operates by binding to thrombin at three critical sites: its active site and two exosites that regulate clotting and platelet activation. This triadic connection yields femtomolar inhibitory binding, far surpassing that of traditional thrombin inhibitors. Contrary to expectations of differing binding patterns due to hybrid composition, XChimera successfully mirrors the original binding interactions of its constituent peptides from leech, fly, and flea.
The synthesis of the hybrid peptide involved the amalgamation of solid-phase peptide synthesis, chemical ligation, and follow-up sulfation processes. Researchers utilized x-ray crystallography to affirm the simultaneous binding of all three thrombin sites. Subsequent biochemical testing and clotting evaluations confirmed the hybrid’s robust thrombin inhibition and its efficiency in preventing platelet aggregation.
Expert commentary on this research underscores the inhibitor’s dual functionality against thrombin’s proteolytic activity and platelet aggregation. Nicolas Winssinger praises its novel activity profile, indicating potential breakthroughs in the creation of safer anticoagulants.
In animal studies, XChimera demonstrated a marked ability to hinder clot formation and diminish thrombus size without causing immediate toxicity. However, progressing to human clinical trials is viewed as an ambitious, long-term objective.
Jeffrey Weitz acknowledges the ingenious chemistry underpinning XChimera but raises concerns regarding potential safety risks linked to its strong irreversible inhibitory functions, given thrombin’s vital role in hemostasis.
Looking ahead, Payne imagines the bioactive peptide hybridization technique being applied to other therapeutic domains, particularly where targeting multiple protein interaction sites is crucial for achieving therapeutic effectiveness.