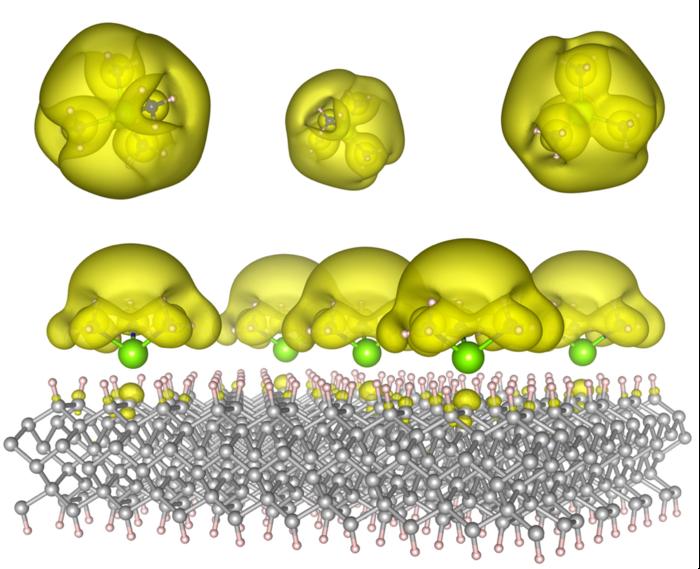
In research facilities at Auburn University, scientists have developed a novel category of materials that could revolutionize both computer calculations and chemical manufacturing processes. The study, detailed in *ACS Materials Letters*, outlines a controllable electride form that allows electrons to traverse a solid surface with ease. These liberated electrons may serve as the basis for enhanced computational speed and improved catalysis efficiency.
Electrides are distinctive solids wherein electrons reside in spaces between atoms rather than being tethered to them. The Auburn researchers achieved a stable variant by attaching unique molecules known as solvated electron precursors to resilient materials such as diamond and silicon carbide. The outcome is a structural entity referred to as a Surface Immobilized Solvated Electron Precursor Electride, or SISEPE, where electrons can be configured like islands or spread out into expansive metallic seas.
## Designing Free Electrons for Practical Applications
Typically, in most materials, electrons are restricted to orbiting particular nuclei. This restriction hampers the material’s ability to conduct electricity, engage with light, or facilitate chemical reactions. The Auburn team aimed to liberate the electrons and manage their movement through meticulous design. By employing cutting-edge computational models, they forecasted the necessary adjustments to the molecules, surfaces, and electron densities to engineer specific properties.
> “By mastering the control of these free electrons, we can engineer materials that perform functions beyond natural capabilities,” expressed Dr. Evangelos Miliordos, Associate Professor of Chemistry at Auburn University and lead author of the study.
Lower-density configurations of the molecules result in localized electron clusters that could function as quantum bits for computational tasks. In contrast, higher-density arrangements convert the surface into a metallic layer capable of efficient charge transfer, perfect for catalytic processes or energy transformation. This same material platform can transition between these different states depending on its fabrication method, providing exceptional versatility for both scientists and engineers.
In the realm of quantum computing, such control could furnish the stability necessary for information processing using electron states rather than conventional transistors. Within the chemical sector, it may enable the creation of catalysts that accelerate reactions while minimizing energy consumption. The results intertwine principles from chemistry, physics, and materials science into a cohesive framework for electronic innovation.
## Stable Foundations for the Quantum Era
Former versions of electrides were often unstable, quickly deteriorating when subjected to air or heat exposure. By securing solvated electron precursors onto solid surfaces, the team from Auburn has illustrated a design that is both durable and adjustable. This method transitions electrides from delicate anomalies to viable materials that can be produced and incorporated into future technologies.
> “Our research opens a new avenue for materials that not only provide opportunities for fundamental studies on interactions within matter but also offer practical implementations,” remarked Dr. Marcelo Kuroda, Associate Professor of Physics at Auburn University.
Coauthor Dr. Konstantin Klyukin, Assistant Professor of Materials Engineering, highlighted the wider implications of this method. By anchoring molecular complexes to precisely defined surfaces, researchers can investigate the behavior of free electrons under varied conditions. It also paves the way for breakthroughs in redox chemistry and the creation of nanodevices. “This is foundational science, yet it has very tangible consequences,” Klyukin mentioned in the university announcement.
The initiative saw contributions from graduate students Andrei Evdokimov and Valentina Nesterova and received backing from the U.S. National Science Foundation. All contributors are affiliated with Auburn’s Center for Multiscale Modeling of Materials and Molecules (CM4), which combines computational modeling with empirical materials research. Within CM4, teams devise new algorithms and simulations that connect atomic actions and macroscopic behavior.
The possible applications are remarkable. Envision computational devices that harness and manage data via the intrinsic quantum features of electrons, or catalytic systems engineering cleaner fuels by minimizing byproduct reactions. Each relies on mastering the dynamics of electron movement, interactions, and coupling with matter. Auburn’s breakthrough offers a framework for achieving just that.
“This is merely the outset,” stated Miliordos. “By understanding how to harness free electrons, we can envision a future with accelerated computing, more intelligent machines, and innovative technologies we have yet to conceive.”
[ACS Materials Letters: 10.1021/acsmaterialslett.5c00756](https://doi.org/10.1021/acsmaterialslett.5c00756)
**There’s no paywall here**
*If our reporting has informed or inspired you, please consider making a donation. Every contribution, no matter the size, empowers us to continue delivering accurate, engaging, and trustworthy science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.*
Join us in making knowledge accessible and impactful. Thank you for standing with us!