**Enhancing the Effectiveness of Fischer–Tropsch Method with Novel Catalytic Techniques**
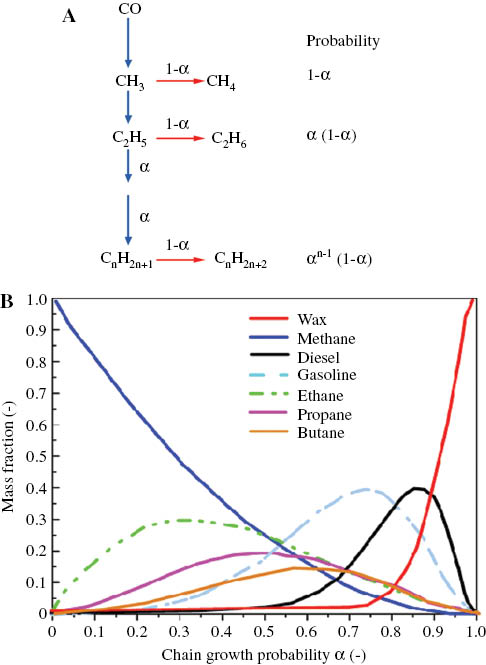
The Fischer–Tropsch reaction, a fundamental pillar of industrial chemistry over the last hundred years, has encountered difficulties pertaining to efficiency and ecological impact. This method, which merges hydrogen and carbon monoxide to generate hydrocarbons, suffers from side reactions that convert a substantial amount of carbon feedstock into CO₂, depleting valuable hydrogen and diminishing overall efficiency. Recently, two groundbreaking catalytic techniques from research groups in China exhibit potential for addressing these challenges.
### Halogen Engagement: Reducing Carbon Dioxide Formation
In one tactic, concealed halogens emerged as crucial in decreasing undesirable byproducts. Researchers from the Chinese Academy of Sciences, spearheaded by Xing-Wu Liu and Yi Cai, discovered through their experiments that trace amounts of halogens on an iron carbide catalyst significantly lowered CO₂ output. By incorporating halomethanes, particularly bromomethane, they demonstrated that as much as 85% of carbon dioxide production could be mitigated, alongside a significant rise in olefin generation.
Their investigations showed that bromine ions obstruct vital stages in the side reactions, specifically the water–gas shift and Boudouard reactions, resulting in diminished water and CO₂ production. This strategy is feasible for current industrial operations and highlights the potential to transform production efficiency while lessening environmental effects.
### Redirection of Reactions: Optimizing Hydrogen Use
Conversely, Chaojie Cui’s research team at Tsinghua University suggested augmenting the hydrogen efficacy of the Fischer–Tropsch process. Rather than inhibiting the water–gas shift reaction, they created a core–shell catalyst system designed to maximize hydrogen utilization. An iron carbide core catalyzes the primary syngas-to-olefin reaction, while an iron oxide shell facilitates the water–gas shift, cyclically regenerating hydrogen within the catalyst framework.
This spatial arrangement holds promise for optimizing hydrogen usage from the feedstock, permitting a reduced hydrogen-to-carbon monoxide ratio and minimizing waste. Nevertheless, additional development is required to guarantee its scalability and long-term viability in industrial settings.
### Prospective Pathways
Both approaches present encouraging directions for enhancing the sustainability of Fischer–Tropsch technology by lowering CO₂ emissions and boosting hydrocarbon production. They signify a notable advancement towards more sustainable chemical methods, potentially paving the way for a future in which green hydrogen drives entirely sustainable hydrocarbon creation. Ongoing research and development initiatives are vital for converting these laboratory achievements into industrial realities, ultimately aiding in the reduction of the ecological footprint of this essential chemical process.