Overview of CRISPR
CRISPR is an innovative tool that is changing lives and creating a stir in the healthcare sector. CRISPR, short for “Clustered Regularly Interspaced Short Palindromic Repeats,” is present in prokaryotes—tiny, unicellular organisms that lack organelles. These sequences are found within the genomes of prokaryotes, which organize a cell’s DNA into bundles.
As a tool for gene modification, CRISPR is utilized to alter specific DNA segments to address serious health conditions. It is especially effective in aiding the body’s defense against viral attacks by integrating the foreign DNA into its own genome, thus enabling it to identify and eliminate the virus in subsequent encounters. Notably, unlike other gene editing techniques, CRISPR is significantly more accurate and simpler to program, which facilitates an easier process of sequence redesign. While other gene-editing instruments generally rely on a single protein, CRISPR employs RNA-guided targeting together with the Cas9 enzyme.
The Mechanism of CRISPR
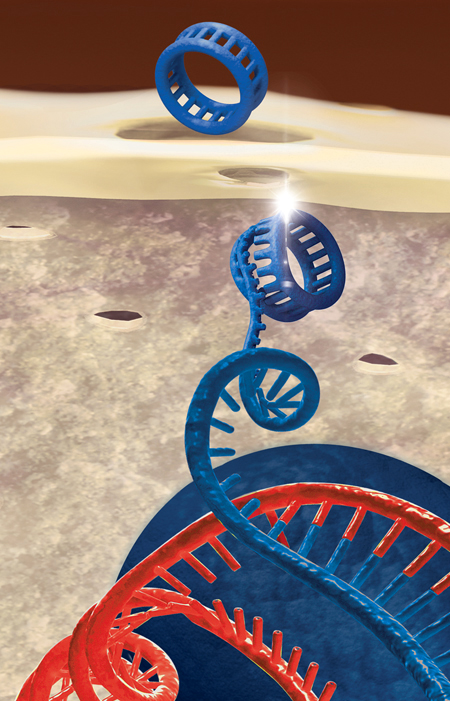
CRISPR operates by utilizing a natural defense strategy found in bacteria that enables them to detect and eliminate viral assailants. When a virus infiltrates a bacterial cell (a prokaryote), the bacterium keeps a fragment of the invader’s DNA within its own genome as a genetic “memory.” This allows the bacterium to recognize and react more adeptly to future invasions.
In gene editing, this system is modified using two essential elements: the Cas9 enzyme, which acts like molecular scissors to cleave DNA, and guide RNA, which steers Cas9 to the precise genetic sequence that requires modification. Once the target DNA is severed, the cell’s inherent repair mechanisms take charge, permitting researchers to introduce alterations to the genetic code.
Unlike previous instruments that depended on complex-to-reprogram proteins, CRISPR’s RNA-guided approach is more adaptable, simpler to design, and extremely accurate. This ease and precision have enabled CRISPR to be utilized in medicine, agriculture, manufacturing, and microbiology—such as altering microbes to enhance product yields. However, as this article discusses, with broader capabilities arise significant ethical dilemmas, particularly surrounding germline editing and genetic enhancement.
First Patient to Receive Personalized CRISPR Therapy
In February 2025, the inaugural personalized CRISPR therapy was administered to an infant named KJ to address a deficiency in Carbamoyl Phosphate Synthetase 1 (CPS1)—an enzyme integral to converting ammonia (produced during protein metabolism) into urea. A group led by Dr. Rebecca Ahrens-Nicklas and Dr. Kiran Musunuru at the Children’s Hospital of Philadelphia created this therapy after extensive research in gene editing and collaboration with other medical professionals.
Their research concentrated on conditions impacting the urea cycle, which results in harmful ammonia accumulation, resulting in damage to organs, including the brain and liver. They customized the treatment specifically for KJ’s type of CPS1 deficiency using prior research on comparable variants.
Until this point, the only CRISPR therapies sanctioned by the U.S. FDA have been for more prevalent illnesses such as sickle cell disease and beta thalassemia, which affect tens or hundreds of thousands of patients. For KJ, his therapy was formulated within six months of his birth, focused on his unique CPS1 variant. The team devised a base editing treatment delivered through lipid nanoparticles to his liver to rectify the defective enzyme.
The February therapy was the first of three doses; KJ received the subsequent two in March and April 2025. As of his last treatment, he has reported no serious adverse effects, demonstrates improved tolerance to dietary protein, and requires reduced medication to regulate ammonia levels. While ongoing monitoring is essential, Ahrens-Nicklas states that the results thus far are encouraging.
CRISPR’s Ethical Implications
Like any pioneering technology, CRISPR prompts intricate ethical considerations. Although its main aim is to modify somatic cells for disease treatment, it can also be applied to gametes, entering the contentious domain of germline editing. Modifying DNA that will be passed down to future offspring is frequently regarded as unethical—especially when performed for the enhancement of traits rather than for therapeutic purposes.
In light of these concerns, researchers have temporarily halted germline editing until its ethical and societal effects are more thoroughly comprehended. The unpredictability of genetic modifications and the lasting nature of these adjustments across generations raise crucial questions regarding the boundaries of this technology.