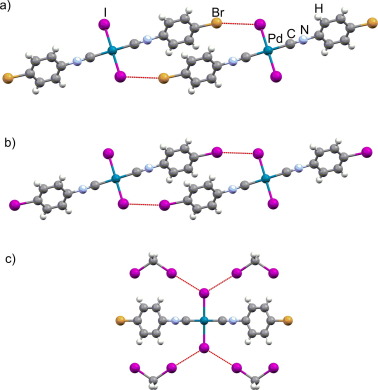
Researchers in Czechia have developed a novel approach to evaluate the covalency of halogen bonds.1 By demonstrating the extent to which electrons are shared in this context, the approach will aid ongoing discussions regarding the physical nature of these increasingly significant supramolecular interactions.
Similar to the more familiar hydrogen bonds, halogen bonds play a key role in the areas of catalyst, drug, and materials design. They occur between distinct molecules or within a single molecule when a nucleophilic area, like a lone electron pair, on one atom is attracted to the electrophilic region, the s hole, on a halogen atom. These interactions hinge on the polarisability and electronegativity of the halogen (X) along with the electron-withdrawing ability of the group to which the halogen is connected (R).
Even though halogen bonds are recognized as electrostatically-driven noncovalent interactions, ‘there’s now plenty of evidence indicating a covalent contribution,’ notes Robin Perutz, an inorganic chemist at the University of York in the UK, who was not part of the study. Typical investigations into the covalency of these interactions involve analyzing crystal structures using techniques such as x-ray diffraction or infrared (IR) spectroscopy. These methods reveal the variation in R–X bond length induced by halogen bonding.2
Currently, Radek Marek at Masaryk University and his team suggest employing paramagnetic nuclear magnetic resonance (NMR) spectroscopy as an alternative approach to explore the covalent characteristics of halogen bonds. ‘This method offers high sensitivity and detailed insights surpassing conventional experimental techniques like x-ray diffraction or IR spectroscopy,’ states Marek.
Marek’s team analyzed the 13C NMR spectra of halogen-bonded cocrystals with either paramagnetic or diamagnetic metal complexes. They observed a significant shift in the peak associated with the carbon directly connected to the halogen (C1) between the paramagnetic and diamagnetic metal cocrystals. This hyperfine shift arises from various interactions between nuclear and electron spins, including the Fermi contact interaction.
‘The Fermi contact contribution to the NMR shifts, related to the intermolecular electron spin transmission [of the metal] to the probe nucleus [C1], serves as an excellent indicator of electron sharing in … halogen-bonded cocrystals,’ clarifies Marek.
‘Our research offers direct, highly sensitive experimental evidence for covalency in halogen bonds,’ asserts Marek. Although non-covalent interactions remain the primary characteristic of halogen bonding, Marek’s group has demonstrated that covalent interactions, i.e., electron sharing, can account for as much as 25% of the total interaction energy.
‘The method is quite impressive,’ remarks Perutz, yet ‘there’s potential for further exploration.’ Given that hyperfine shifts fluctuate with temperature, Perutz expresses surprise that the team did not examine the temperature dependency of paramagnetism to exclude other competing factors. He suggests additional NMR investigations focusing on adjacent bonded fluorines, which could unveil even greater covalent intricacies.
Perutz also raises the question of whether more sensitive techniques already exist, mentioning that ‘there are other situations where you can achieve significantly larger shifts’.2,3
Nonetheless, both Marek and Perutz agree that enhancing our understanding of halogen bonding is crucial for improving accuracy in modeling the interactions of catalysts, functional materials, and pharmaceuticals.