Researchers have achieved a remarkable milestone by observing the inaugural stable germa-isonitrile bond, overcoming a problem that has endured for more than 160 years. Traditionally, isonitriles (R–N≡C) have served as crucial elements in chemistry owing to their durability and adaptability. However, synthesizing heavier group-14 counterparts, referred to as tetrela-isonitriles (R–N≡E, where E stands for Si, Ge, Sn, Pb), has proved challenging, with these N≡E triple bonds manifesting only as transient intermediates in cryogenic or gas-phase investigations. The difficulty stems from the fact that heavier elements enhance the bond’s polarity and weaken π-bonding, leading to aggregated molecular structures instead of solitary molecules.
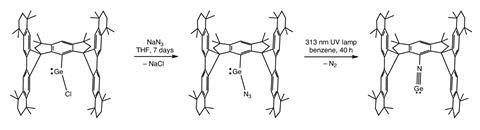
A team of researchers from China has now successfully isolated a stable germa-isonitrile (Ar–N≡Ge), employing an exceptionally bulky aryl ligand to preserve the bond’s stability. This success drew inspiration from prior research indicating that crowded hydrindacene-based ligands could stabilize reactive low-valent main-group species. The team’s approach relied on applying steric hindrance to circumvent the common oligomerisation problems associated with tetrela-isonitriles, successfully isolating a stable monomer.
X-ray crystallography validated the existence of a genuine N≡Ge triple bond at ambient temperature, stabilized by the large ligands. Additional solid-state NMR and computational studies indicated that the Ge–N bond is remarkably short and highly polarized, with a bond length of 1.64Å. This polarity enhances its reactivity, permitting the germa-isonitrile to interact with organic electrophiles and transition-metal complexes, thus opening avenues for new chemical reactions and applications previously considered impossible.