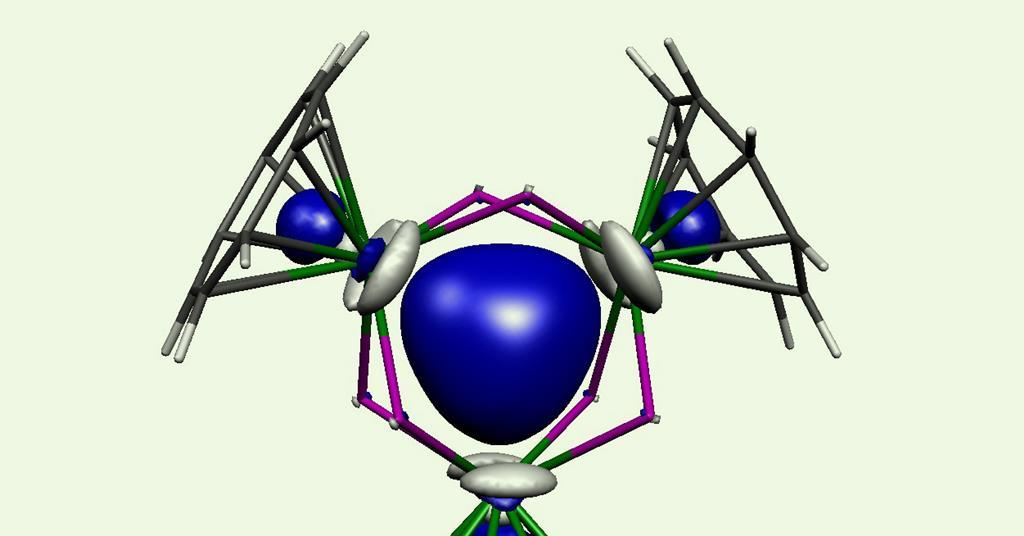
A dark blue compound with a central trithorium ring is the first σ-aromatic actinide compound ever created. Its discovery challenges theoretical calculations that have predicted actinide–actinide bonding to be weak and localised rather than the strong delocalised bonding found in aromatic compounds.
While actinides like thorium and uranium have been introduced into aromatic organic compounds, this is the first example in which the aromatic ring is made entirely out of actinide atoms. The molecule is also the first featuring any type of actinide–actinide bond that has been isolated under normal conditions rather than at ultracold temperatures or in the gas phase. The molecule was created by reacting a half-sandwich cyclooctatetraenyl thorium complex with a strongly reducing potassium cyclobutadienyl compound.
Calculations of the molecular orbitals, electron density distribution and bond indices revealed a delocalised three-centre two-electron σ-aromatic bond in the compound’s trithorium core. Moreover, the molecule’s nuclear independent chemical shift values – a popular way to measure aromaticity – was found to be similar to other two-σ-electron aromatics like the lithium cluster cation Li3+. The three-centre two-electron bonding pattern has been seen in main group and transition metals before, but it’s a first for heavy elements.