The first experimental images of a bromine atom’s σ-hole have been unveiled, mapping the peculiar charge distribution that is the basis for halogen bonding.
Although it seems counterintuitive that two negatively charged atoms attract rather than repel each other, this is the case when it comes to halogen bonding. Halogen atoms form attractive intermolecular bonds with other halogens, as well as electron donors such as oxygen. Halogen bonds – variations of which also exist in elements of other groups – are essential for holding together supramolecular crystals and even biological macromolecular structures.
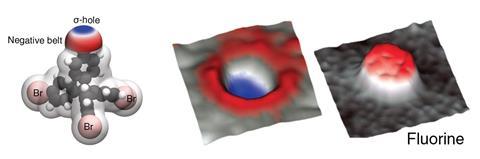
Key to halogens’ ability to form these bonds is their σ-hole. This is an area of low electron density – and therefore partial positive charge – on the opposite side, but along the same axis, to where the halogen is bound to another atom. So far, the only visualisations of the σ-hole came from indirect quantum simulations or crystal structures.
Now, researchers from the Czech Republic have snapped the first real images of a bromine atom’s σ-hole. The team put a single xenon atom onto the tip of a Kelvin probe force microscope, a non-contact variant of an atomic force microscope. This allowed them to image the σ-hole in one of tetrakis(4-bromophenyl) methane’s bromine atoms. They compared it with a fluorine version of the same molecule, which didn’t show a σ-hole.
The team suggests that, apart from providing the first direct evidence for the existence of σ-holes, their method could image charge distribution in other complex molecular systems.