An electrocatalytic method has been developed that can generate metal hydrides more cleanly and efficiently. The researchers then used the hydrides to reduce carbon dioxide to formic acid, a high value-added commodity chemical.
‘We were inspired by the efficiency of enzymes,’ explains Victor Mougel from ETH Zurich in Switzerland, who led the study. In nature, many different enzymes catalyse proton-electron transfers, the type of reactions that yield hydrides. ‘In particular, an iron–iron hydrogenase that follows a concerted mechanism … it transfers both a proton and an electron together … to generate the metal hydride,’ he says. And it works thanks to one of the most common catalytic cores in living organisms – the iron–sulfur cubic cluster. Mougel’s team maintained this motif as a mediator.
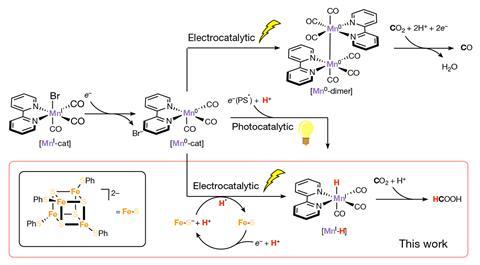
‘For the first time … it’s possible to electrochemically generate a reactive metal hydride tuning the properties of the iron–sulfur cluster,’ says Eva Nichols, an expert in bio-inspired electrocatalysis at the University of British Columbia, Canada. ‘Metal hydrides are useful in reduction reactions.’ More familiar examples include sodium borohydride and lithium–aluminium hydride. In this case, the iron–sulfur cluster promotes the electrocatalytic formation of a manganese hydride. Nichols explains that ‘transition metal hydrides are particularly interesting’, because researchers tune their properties by tweaking the metal centre, its oxidation state or the organic ligands around it. ‘This [manganese] hydride reduces carbon dioxide into a valuable product: formate,’ she says.
The key, explains Mougel, was mimicking the mechanism. Traditionally, generating hydrides requires the recurrent reduction of the metallic centre, then capturing a proton. Such stepwise redox reactions take place ‘through high-energy intermediates, [which need] either harsh acids or extreme negative potentials’, explains Nichols. Moreover, they create very reactive metallic intermediates, which bind to other products present, instead of picking protons up to produce hydrides. ‘The manganese complex we used, for example, reacts with carbon dioxide almost 20 times faster,’ adds Mougel. This favours the formation of carbon monoxide, but hinders hydride generation.
Among the many mediators tested, the iron–sulfur cluster gave the best results in terms of efficiency and selectivity. ‘It’s good for several reasons,’ explains Nichols. It showcases an appropriate affinity – the perfect bond strength to hold onto the hydrogen atom, and later transfer it to the manganese complex. ‘The iron–sulfur cluster is also very rigid, which helps increase the [reaction] rates,’ she adds. It also reorganises and recovers its original redox state efficiently, which boosts recyclability. ‘The iron–sulfur cluster used as mediator regenerates really quickly, that’s probably why it has naturally evolved in enzymes,’ says Mougel.
‘When compared to [other] catalysts, ours offers high turnover at very low potential,’ he says. The process also has a significant selectivity towards formic acid, ‘one of the most interesting commodity chemicals derived from carbon dioxide’, according to Mougel. The paper also provides a protocol to better choose mediators and metal hydrides. ‘These rules were probably evident to everyone in the electrocatalysis field, but we could test them experimentally – and now they’re verified,’ he says.
Usually, electrocatalysts enable the formation of value-added products using energy from sustainable sources, such as solar and wind. Additionally, ‘[this] concerted approach relaxes the reliance on harsh, hazardous chemicals’, comments Nichols. ‘It’s an impactful inspiration … similar processes could [catalyse] many other reductive transformations in the field of electrocatalysis.’