Researchers have developed a method for preparing silanols that uses an earth abundant metal centre and generates minimal waste products.1
Silanols are compounds containing an Si–O–H functional group, and have applications as both building blocks in synthetic chemistry and materials science. They are easily prepared from silanes through an oxidation reaction, which involves breaking an Si–H bond and forming a new Si–O bond.
While oxidising silanes to silanols is normally a thermodynamically-favoured process, in practice the reaction is slow. Catalysts can accelerate the transformation, but effective catalysts normally contain precious heavy metals2 or expensive ligands.3 Furthermore, the oxygen atom often needs to be provided by an external oxidising agent, which generates waste.4
Now, researchers led by Christophe Werlé and Walter Leitner at the Max Planck Institute for Chemical Energy Conversion in Germany have shown that a simple manganese catalyst is adept at performing this transformation, using water as an oxidising reagent. The only side-product of the reaction is hydrogen.
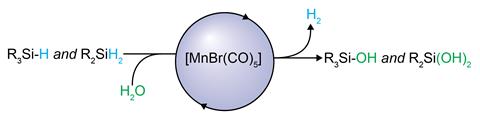
‘Traditionally, organic matter is oxidised using stoichiometric quantities of chemical oxidants like peroxides, metallic salts or oxygen,’ explains Werlé. ‘As a result, functional groups that are prone to oxidation are not tolerated.’ The team used a commercially available MnBrCO5 complex, which performed the transformation rapidly at low temperatures across a range of substrates. Compared to precious metals such as platinum and palladium, manganese is more abundant and less toxic, making it a desirable candidate for performing these kinds of transformations.
The presence of carbonyl ligands on the manganese centre meant the catalytic mechanism could be investigated through Fourier transfer infrared spectroscopy analysis of the C–O bond and 13C isotopic studies. Through this and further kinetic and spectroscopic investigation, the researchers proposed the key catalytic intermediate is a cationic [Mn(CO)3R3]+ species, which readily coordinates with the silane Si–H bond to instigate the reaction.
David Lacy of Buffalo University also uses manganese complexes for sustainable transformations and has investigated the mechanistic behaviour of similar manganese species in catalysis.5 He calls it a well-executed study, and says ‘it is nice to see that simple organomanganese compounds are finding use in synthesis.’
The team hope that their reaction, and the synoptic approach to studying the catalytic mechanism, will be useful to other researchers. ‘We will be interested to see whether our new insights can lead to the design of future manganese catalysts capable of similar organometallic elementary steps and challenging bond-breaking and -forming processes,’ adds Werlé.
References
1 E Antico et al, Chem. Sci., 2023, DOI: 10.1039/d2sc05959b
2 J Li et al, ChemistrySelect, 2021, 6, 8345 (DOI: 10.1002/slct.202101241)
3 W Yang et al, Angew. Chem., Int. Ed., 2022, 61, e202205743 (DOI: 10.1002/anie.202205743)
4 K Valliant-Saunders et al, Inorg. Chem., 2007, 46, 13, 5212 (DOI: 10.1021/ic062468u)
5 P C Abhyankar, S N MacMillan, D C Lacy, Chem Eur. J., 2022, 28, e202201766 (DOI: 10.1002/chem.202201766)