A new organocatalyst could cut the energy use of a vitally important chemical process – the chlor-alkali process. Yadong Li from Tsinghua University in China, and his team found that relatively cheap organic quinazoline-2,4-diones reduce the energy consumption of the process compared with expensive metal catalysts. If such a catalyst were adopted worldwide by the chlor-alkali industry, it might save approximately 1.8–4.6% of the 150TWh it consumes each year, Li’s team finds. That would roughly equate to between three to nine days of average daily UK energy consumption. Other scientists say the catalyst’s stability still needs a lot of work before such energy savings could be realised.
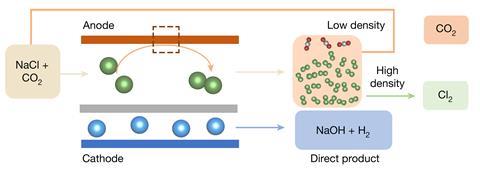
The chlor-alkali process passes electricity through a sodium chloride solution, generating chlorine and sodium hydroxide. The European Commission has estimated that this process underpins more than half of the continent’s chemical industry sales, with 2 million jobs linked to its products. The chlor-alkali process’s importance is matched by its energy intensity, as it consumes around 4% of the electricity the world produces.
Traditionally, the chlor-alkali process happens in membrane cells, reactors with two chambers, each containing an electrode. Electrical current passes through a concentrated salt solution in the first chamber, which contains a dimensionally stable anode (DSA), releasing chlorine. The DSA is usually titanium coated with a conductive oxide of a noble metal like ruthenium and also corrosion-resistant titanium dioxide. The other chamber separates water into hydrogen and hydroxide ions at the cathode. Sodium ions move from the first chamber to the second and combine with hydroxide ions to make sodium hydroxide.
Harsh environment
Industrial efforts to make the chlor-alkali process up to 25% more efficient are already happening. This involves adding oxygen to produce more hydroxide ions from much of the hydrogen gas that would otherwise be released. This reaction changes the electric potential at the cathode, reducing the potential difference between electrodes. That lowers the membrane cell’s voltage, and therefore its energy consumption. This is the process that Li and his team have now sought to further improve.
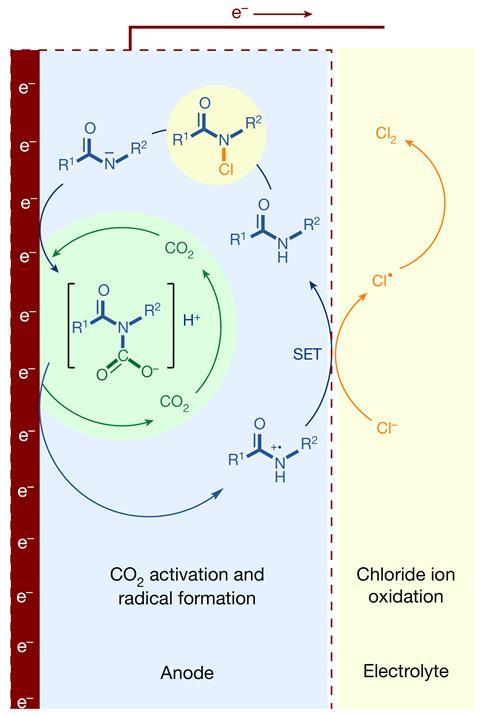
Li’s team replaced the DSA with organic molecules containing amide groups supported on an electrode made of titanium and carbon. They also bubble carbon dioxide through the cell, which reacts with the amide compounds to produce carbamic acid intermediates, enabling catalytic cycles that produce chlorine with great efficiency. One key challenge with this new catalyst is that its activity slowly decays with use, although the team believes that a better preparation method might prevent this.
Rolf Hempelmann from the University of Saarland calls the study ‘a nice piece of academic work’ but doubts its industrial relevance. ‘Chlorine gas at oxidising electrochemical potential is the harshest chemical environment one can imagine,’ he says. The way that the catalyst system is currently constructed cannot compete with the 10-year lifespan of those that are currently used, he asserts.
‘It will be very difficult to achieve the required durability over several years,’ agrees Thomas Turek from the Technical University of Clausthal, who wrote a news and views article to accompany the new study. But he doesn’t believe that it is impossible. ‘The great value of the publication, in my view, is that for the first time an organic catalyst with these excellent properties for chlorine evolution has been demonstrated,’ he adds. ‘It is a first step, and obviously further research effort is required.’