For the first time, researchers have monitored a solute ion binding to its surrounding solvent. The experiments could offer a route towards being able to track important transformations like cation–molecule complex formation in real time.
A team led by Henrik Stapelfeldt from Aarhus University, Demark, designed an experiment to follow what happens as a sodium ion dissolves in a droplet of liquid helium. The process begins with a single sodium atom sitting in a dimple on the droplet’s surface, while a xenon atom is contained in the droplet’s interior. The team trigger the solvation process by using a femtosecond laser pulse to ionise the sodium atom – helium atoms are now attracted to the positively charged sodium ion and gradually bind to it. A second laser pulse then ionises the xenon atom. The positively charged xenon ion now repels the sodium–helium complex causing it to be ejected from the droplet. Detectors allow the team to monitor the ejected complexes and determine how many helium atoms are bound to the sodium ion. By varying the time between the first and second laser pulses the team gained insight into the rate at which the solvent binding process takes place.
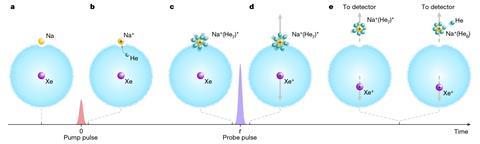
The experiments showed that the first five helium atoms bind to the sodium ion at a rate of two atoms per picosecond (one trillionth of a second). Stapelfeldt’s team believes that the findings make it possible to experimentally benchmark theoretical models of ion solvation and opens the door to measuring the solvation dynamics of other alkali and alkaline earth ions.