A few days ago a company called Freedom Industries spilled a massive amount of a licorice-scented compound called 4-methylcyclohexane methanol into the Elk River, contaminating local water supplies and forcing authorities to turn off the taps until they can determine the extent of the problem. For folks who live in the area, this must be very frustrating — all they’ve been told is that they can’t drink the water and they don’t know when it will be back online, so the bottled water sold out overnight.
The spill has a lot of people wondering: what exactly IS 4-methylcyclohexane methanol (I’ll call it 4-MCMH) and how dangerous is it? Unfortunately the toxicology of 4-MCMH has been poorly studied, so neither I nor the federal gov can be sure exactly how dangerous it is. But let’s see how much we can figure out by looking at the structure of the molecule and thinking about what it’s likely to do inside the body. And as always I’ll try and make this as simple and non-scientist friendly as possible in keeping with the spirit of this blog.
So here’s the structure, courtesy of the wiki: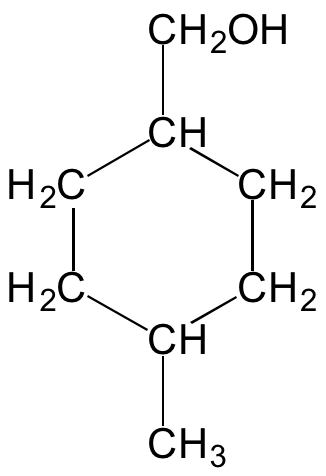
Now this is drawn in 2D, but an actual molecule is a 3D structure. All of these carbons are single-bonded which means each of them is bonded to four other atoms. When a carbon is bonded to four other atoms those bonds tend to be arranged in a tetrahedral shape like this:
![]()
You can explain this tetrahedral shape using a model called molecular orbital theory, which mathematically describes the structure and properties of molecules in terms of quantum mechanics, but that gets very complicated. An easier way to explain why a carbon atom has a shape like this is through a simpler (though completely non-mathematical) model called VSEPR. The idea is like this. A bond between atoms is a pair of shared electrons, right? And electrons are negatively charged, so they repel each other. Therefore bonds to an atom are going to arrange themselves in such a way they are as far apart from each other as possible. A carbon atom with four bonds will therefore tend to have the shape shown above — because that maximizes the distance between all four bonds.
So if you take 4-MCMH and look at it in 3D, you’d find each of the carbon atoms in it would be at the center of a tetrahedron like the one above, which means that six-carbon ring would look something like this:
If that’s what it looks like how come we don’t draw it like that? Well, fact of the matter is most chemists are really crappy artists. We’re the kind of folks who did just fine in calculus and o-chem but failed that stupid art class, you know. So by convention all chemical structures in chemistry are drawn in 2D because otherwise most of us wouldn’t be able to draw them. When we look at a chemical structure drawn in 2D we automatically mentally convert it into 3D — we just can’t draw them like that because we suck at art (unless you give us a computer that can draw for us). Anyhow.
What can we figure out from the chemical structure? Well, we know that water is very polar, meaning electron density in water molecules is very unevenly distributed. (See my earlier post about crack cocaine if you don’t remember why that is). Therefore as a general rule of thumb water is good at dissolving things that a) have a charge on them or b) are very polar. This molecule DOES have a polar OH group so that should help. But the area of the molecule that’s polar is just a small fraction of the area that’s plain old hydrocarbon which is very nonpolar. So you would expect it would dissolve quite poorly in water. You can probably get it into water at low concentrations (“appreciable” is the word in the MSDS, which is sort of vague) but it’s definitely not a very water-friendly compound. It’s also less dense than water so it will tend to float and form a separate layer on top.
You’d also expect the molecule should not be brightly colored. Carbon-based or organic molecules as a general rule of thumb are usually brightly colored if they contain either a) extensive alternating single-double bonds or b) a metal ion with a bunch of other junk stuck onto it. (See previous posts for more on why that is.) This molecule doesn’t have either of those things, and so as you’d expect it’s colorless. Some of the news reports said something about a green color in the water, which if true bothers me a little. (More on that in a minute.)
Is it dangerous? Well, like most organic solvents it’s flammable, but as you’d expect it’s a lot less volatile than other organic solvents like acetone or hexane. As a general rule of thumb, the bigger and/or more polar the molecule the less volatile it will be. That polar OH group on this critter can form weak bonds with other OH groups called hydrogen bonds and that will help to hold them together so they don’t evaporate as readily. Bu what we really want to know is what it does to humans, animals and plants. Unfortunately that’s a lot tougher to figure out.
First off, this chemical is NOT highly reactive, and we can tell that based on the structure. It should not react with either proteins or DNA. Since its water solubility is low, it will probably be pretty good at getting into fatty adipose tissue; it’s going to be pretty fat-soluble. Your liver will probably try to oxidize it to make it more water-soluble, e.g. by converting that H2C-OH group to a COOH group. A COOH group is acidic so it can lose the H and end up with a negative charge, e.g. COO-, which is WAY more water soluble than the OH group because it’s negatively charged now. It’s possible your liver will do other stuff to it as well. Once your liver has altered it a bit it will probably leave your body by way of your urine.
OK, fine. So this doesn’t look too dangerous. But we still don’t know for sure for two reasons. First of all, there are many thousands of proteins in your body. We don’t know what all of them are, and we don’t know what all of them do. There’s always a possibility a compound has just the right shape to stick to Unknown Protein X and thereby cause Dangerous Side Effect Y. Second, it’s always possible your liver will do something weird to this molecule and thereby make it more toxic. I can’t see anything obvious your liver could do to 4-MCHM to make it into a problem but you never know.
The upshot is you can’t tell for sure from a molecule’s structure how toxic it will be. You can spot obvious problem molecules (stuff that will UNDOUBTEDLY be toxic because it’s highly reactive in certain ways), but just because a molecule has no obvious problems doesn’t mean you’re in the clear. You have to do some experiments before you can be absolutely sure, which is how toxicologists and many other biologists make a living.
So what kind of data do we have? Unfortunately not much. This isn’t a widely-used chemical so the folks at FDA and EPA haven’t devoted much attention to it. The LD50 in rats — the amount it takes to kill 50 percent of a population of rats (and yeah, I know, that sounds like a not-very-nice experiment, but it’s one of those necessary things that toxicologists do) is 825 milligrams per kilogram of body weight. Assuming its toxicity in humans is similar, you’d have to drink a lot of this stuff to kill yourself.
The MSDS also lists the no-observed effect concentration in fathead minnows as 25 milligrams per liter (aka ~25 ppm), meaning that concentrations below that show no obvious effects in minnows and such. Now this is a possible problem. I don’t know how much of this stuff got dumped in the river. If we’re talking about a whole tankload, then you could potentially have situations where concentrations in specific spots or areas temporarily shoot high enough to hurt some fish and/or other aquatic organisms. (Fish don’t like swimming around in concentrated organic solvent any more than the rest of us.) But the environmental damage should not be broad or long-lasting because this stuff will break down in the environment. (Once it’s been diluted to low concentrations, bacteria will start munching it for one thing.)
There doesn’t seem to be any data on carcinogenicity. The MSDS says that prolonged exposure may cause a skin rash, and as always when in doubt rubbing it in your eyes is probably not such a great idea.
So to summarize I’d say this (in my decidedly non-medical, non-expert capacity). Don’t drink the water, but if I were me I wouldn’t panic, either. I’ve spilled far more dangerous chemicals on myself and I’m still here. You’re probably in much more danger handling gasoline than handling this stuff, and all of us handle gasoline on a regular basis. (Gasoline DOES contain a little benzene, which is both volatile and a known carcinogen.) ON THE OTHER HAND, in the absence of a detailed toxicological profile I would refrain from drinking or bathing in said water because as discussed we don’t know for sure until the appropriate toxicological studies have been done, and there’s no point being a guinea pig.
The one thing that bothers me a little is this. Like most industrial chemicals this probably isn’t very pure. In the pharmaceutical/biotech industry, when we make a drug for sale or use in clinical trials, we make it about as pure as it can get. And in my subfield of analytical chemistry, I buy & work with very pure reagents because I’m trying to figure out what’s present in a sample and how much of it, so I need to minimize background/contamination, and I can get VERY anal about that, let me tell you. But large-volume industrial chemicals are a different story. Why bother purifying something if you’re just using it to wash coal? come on.
So there could very well be other stuff in there besides 4-MCMH, and it’s always possible there are impurities more nefarious than 4-MCMH itself. As I said, this talk about blue-green water puzzles me because 4-MCMH is colorless, so it’s definitely NOT the 4-MCMH that’s responsible for the color. At least one news report I saw distinctly said the water had turned blue-green. (NOTE: See update/clarification at bottom of post). Perhaps Freedom Industries could clarify. What else is in there? a lot of folks in West Virginia would sort of like to know.
Oh, and I guess the final moral of the story would be this. Don’t situate your chemical plant on the river upstream from a major water treatment plant, especially if you’re making a chemical for which no detailed toxicological profile exists, because if you DO spill something it will make a lot of people very miserable. So no, that’s not really a bright idea…
Update: A commenter pointed out that the Elk River is apparently always blue-green this time of year thanks to algae, so the blue-green “stain” described by the New York Daily News could be normal and have nothing to do with the spill.