A technique that combines two widely used coupling reactions offers a new way to make diaryl amine compounds. The new reaction brings together Suzuki–Miyaura and Buchwald–Hartwig couplings to make carbon–nitrogen–carbon linked products. The researchers behind the work hope that it will enable synthetic chemists to create diverse libraries of compounds that could boost drug-discovery efforts.
While both couplings traditionally employ palladium-based catalysts, Suzuki–Miyaura allows for carbon–carbon bonds to be formed from organohalide and organoboron species, whereas Buchwald–Hartwig amination forms carbon–nitrogen bonds from aryl halides and amines. Now, Richard Liu and coworkers from Harvard University, US, have developed a diverted cross-coupling in which a nitrene insertion reroutes a typical biaryl forming Suzuki–Miyaura reaction to instead produce diaryl amines.
Given the utility and generality of carbonylative transformations, we were surprised to see that insertive cross-couplings involving other “middle components” had been scarcely reported,’ explains Liu. The new three-component coupling reaction works by slowing the highly efficient Suzuki–Miyaura carbon–carbon bond forming process just enough to allow for nitrogen insertion while ensuring that the second carbon–nitrogen bond formation is still possible. The insertion is encouraged via reaction of an electrophilic nitrene reagent with an arylpalladium intermediate. The highly selective reaction generates a variety of unsymmetrical products from a wide range of starting materials including aryl halides, pseudohalides, boronic acids, and esters.
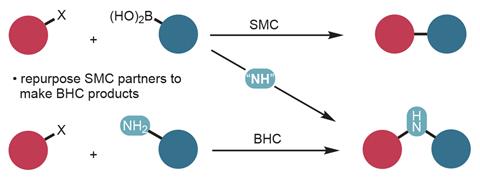
The synthetic reliability of cross-coupling reactions means that biaryl compounds are commonly investigated for their pharmaceutical properties. The new coupling strategy has the potential to broaden structural variety, and could therefore be used to finetune the geometry, polarity and hydrogen bonding of drug candidates, explains Liu, ‘We hope that the method will allow users of cross-coupling to repurpose their Suzuki–Miyaura reactants to make new products,’ he adds. ‘We envision this being used as a tool for generating diversity: for Suzuki–Miyaura couplings, the user now has the opportunity to run a second, parallel reaction that generates the NH-inserted product instead.’
Synthetic chemist Stephen Buchwald from the Massachusetts Institute of Technology, US, who helped to pioneer the Buchwald–Hartwig reaction, is impressed with the new findings. ‘This is a great piece of work by a brilliant young scientist, allowing for a different disconnection for the preparation of aromatic amines,’ he says.
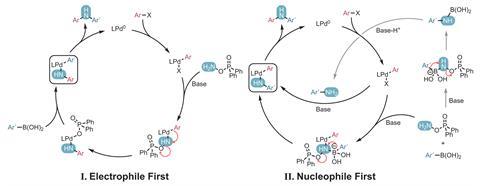
Questions remain over the reaction mechanism and what exactly dictates the order in which the new bonds are made. In some cases, the reaction proceeds by an electrophile-first mechanism, whereby the first carbon–nitrogen bond is formed from the aryl electrophile. However, a nucleophile-first pathway is also possible, and future work aims to explain which route is preferred by a given substrate. ‘What is really puzzling is the reaction mechanism,’ notes Philippe Dauban, an expert in organometallic catalysis from Paris-Saclay University in France. ‘Other hydroxylamine-derived reagents are known to react with boronic acids under transition metal-free conditions to afford the corresponding free anilines, but here the transition metal seems to be required for both carbon–nitrogen bond-forming reactions.’
Olivier Baudoin, an expert in palladium catalysis from University of Basel, Switzerland praises the approach. ‘This new aminative cross-coupling reaction holds great promise for applications in medicinal chemistry where nitrogen atoms are ubiquitous,’ he says. ‘It relies on a clever combination of a sterically hindered phosphine ligand and an ambiphilic amination reagent. The new reaction should have a broad impact in industry.’