In Nature’s Micro War: How Bacteria Employ Protein Filaments to Combat Viral Intruders
In the unseen domains of microbial conflict, an intriguing evolutionary arms race occurs continuously—bacteria versus viruses, or more accurately, bacteriophages. As viruses pursue bacteria with merciless accuracy, researchers are uncovering the intricate defense mechanisms that bacteria deploy. A groundbreaking study has identified a striking molecular strategy utilized by bacteria to starve off invaders by interfering with a crucial molecule necessary for cellular energy and longevity—nicotinamide adenine dinucleotide (NAD+).
Conducted by scientists from Rockefeller University and Memorial Sloan Kettering Cancer Center and featured in the journal Science, the research highlights Cat1, a protein that constructs complex defensive frameworks to cease viral replication by diminishing NAD+. This finding sheds light on the advancing sophistication and variety of tools within the bacterial defensive toolkit—and paves the way for new advancements in biotechnology.
An Alternative Strategy to CRISPR: Transitioning from Cutting to Starvation
Many recognize CRISPR mainly for its gene-editing abilities—a technological breakthrough that originated from our comprehension of bacterial immunity. The CRISPR-Cas9 system, often compared to molecular scissors, provides precise cleavings in DNA and was awarded the Nobel Prize in Chemistry in 2020. However, this represents only one aspect of a more intricate system rooted in microbial evolution.
Introducing Cat1, a part of the CARF effector family (CRISPR-associated Rossmann fold domain proteins). In contrast to their CRISPR-Cas9 relatives, CARF effectors do not directly assault viral DNA. Instead, they disrupt the internal functions of the host cell to make it unwelcoming to the invader through a method of programmed metabolic interference.
Cat1 specifically targets NAD+, a vital molecule for a cell’s metabolism and existence. “Upon sufficient cleavage of NAD+, the cell transitions into a growth-arrest state,” explains Christian Baca, a graduate student and co-first author of the research. By halting metabolic activity, Cat1 obstructs the replication and dissemination of the virus throughout the bacterial community. A population-level sacrifice—altruism on a single-cell scale.
The Molecular Framework of a Defense Network
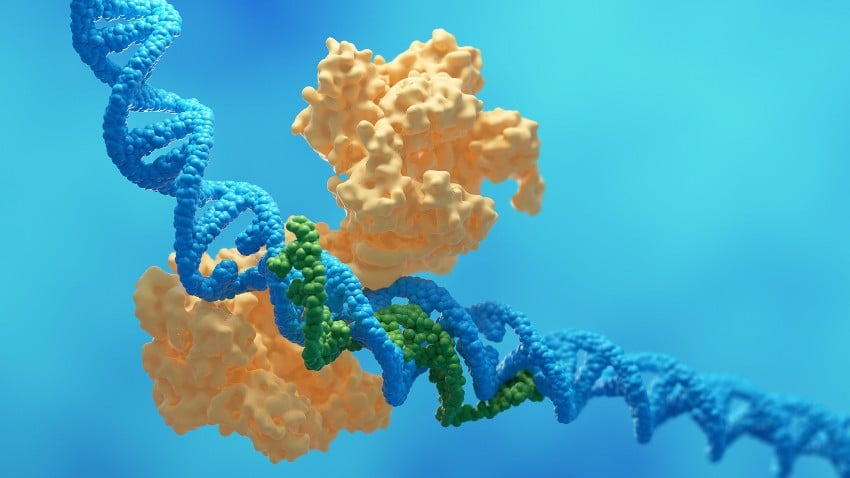
Utilizing cryo-electron microscopy (cryo-EM)—a sophisticated imaging technique that reveals molecules at near-atomic detail—the researchers uncovered the elaborate configuration Cat1 assumes under attack conditions. When activated, the protein assembles into complex, multidimensional filaments and bundles.
Co-first author Puja Majumder from Memorial Sloan Kettering refers to these arrangements as “trigonal spiral bundles” that can develop into even more intricate “pentagonal spiral structures.” These formations are not mere random clumps; they are meticulously organized to optimize their antiviral effectiveness.
Molecular Origami: The Mechanism of Activation
However, Cat1 is not always operating at full capacity. Its activation is finely regulated by a molecular signaling network that ensures it only shifts into defense mode during an authentic viral assault. Central to this process is cyclic tetra-adenylate (cA4), a small molecule produced when CRISPR systems identify viral genetic material.
cA4 binds to Cat1 protein units, prompting them to connect and form their antiviral filaments. These cA4 molecules literally keep the protein components together, allowing the structure to expand like an architectural framework in times of crisis. It is the microbial equivalent of dispatching firefighters only when a fire is confirmed.
An Altruistic Defense: Sacrificing for Survival
The study reveals a deeper insight into bacterial behavior as more than mere primitive survival. The utilization of Cat1 represents a deliberate sacrifice—slowing or halting cell growth to diminish the likelihood of viral replication, thus safeguarding the broader bacterial community. Time-lapse microscopy even demonstrated that cells infected but utilizing Cat1 ceased growth while neighboring cells remained unaffected.
Interestingly, this process isn’t necessarily lethal. Cells can recuperate once the viral threat subsides and NAD+ levels return to normal—unlike certain bacterial defenses that result in cell self-termination. This “hibernation mode” aligns with a growing category of microbial strategies focused on resource denial instead of complete self-destruction.
Broadening the Bacterial Arsenal
Cat1 is a component of a broader toolkit of CARF effectors. Additional known CARF proteins trigger membrane depolarization or release toxic metabolites to create inhospitable conditions during infections. However, what distinguishes Cat1 is its apparent independence.
While many CRISPR systems depend on multiple proteins working collaboratively for defense, some bacteria rely solely on Cat1—and still execute an effective antiviral response. As Christian Baca notes, “Most of the bacteria that encode Cat1 seem to primarily depend on Cat1 for their immunity effect.” This indicates that NAD+ depletion may represent one of the most potent countermeasures in bacterial defense.
Consequences for Science and Technology
Beyond the captivating biology, revelations like this broaden the scope of bioengineering and synthetic biology. Grasping how proteins such as Cat1 function can lead to groundbreaking advancements.