**Introduction to CRISPR**
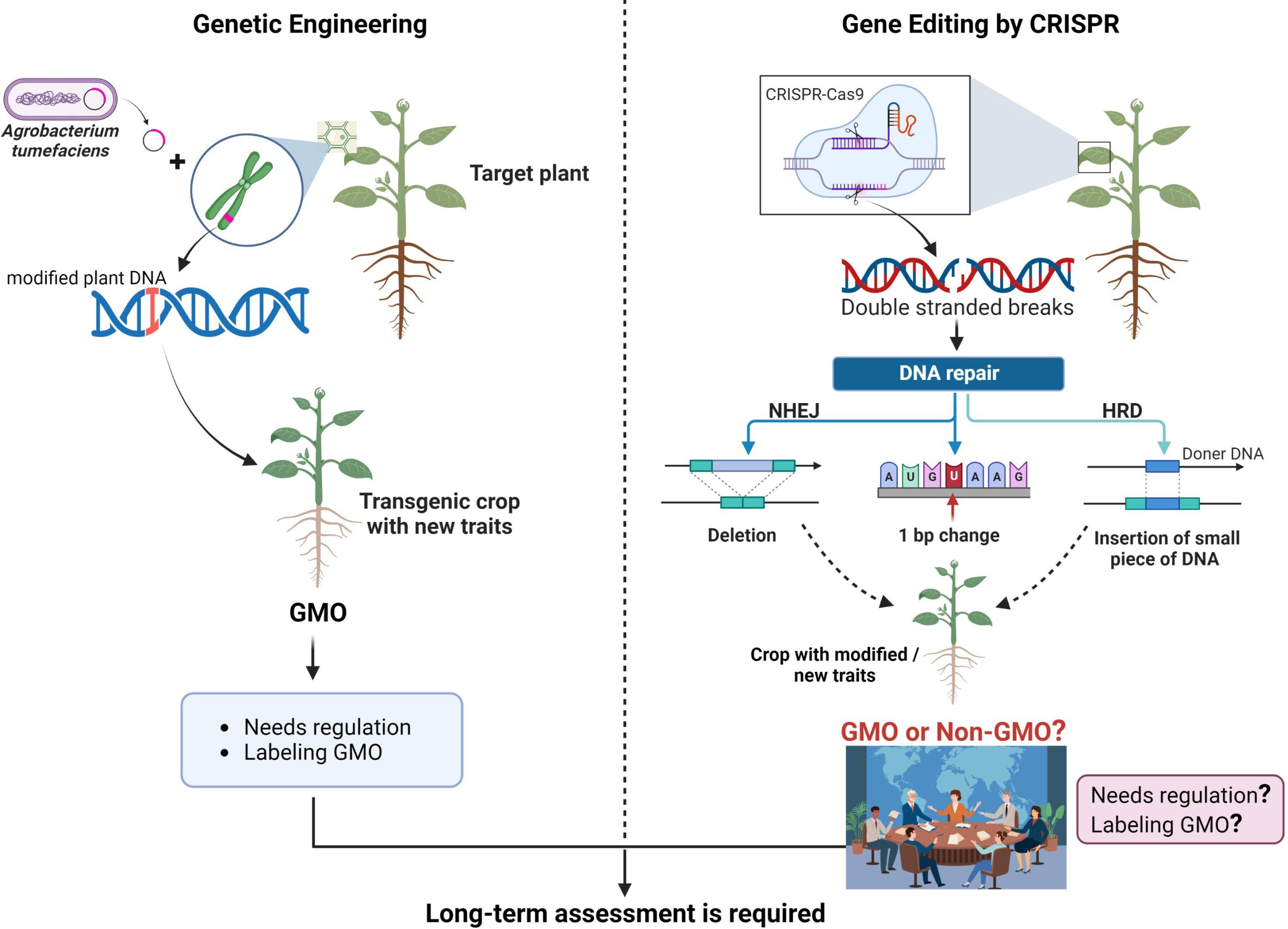
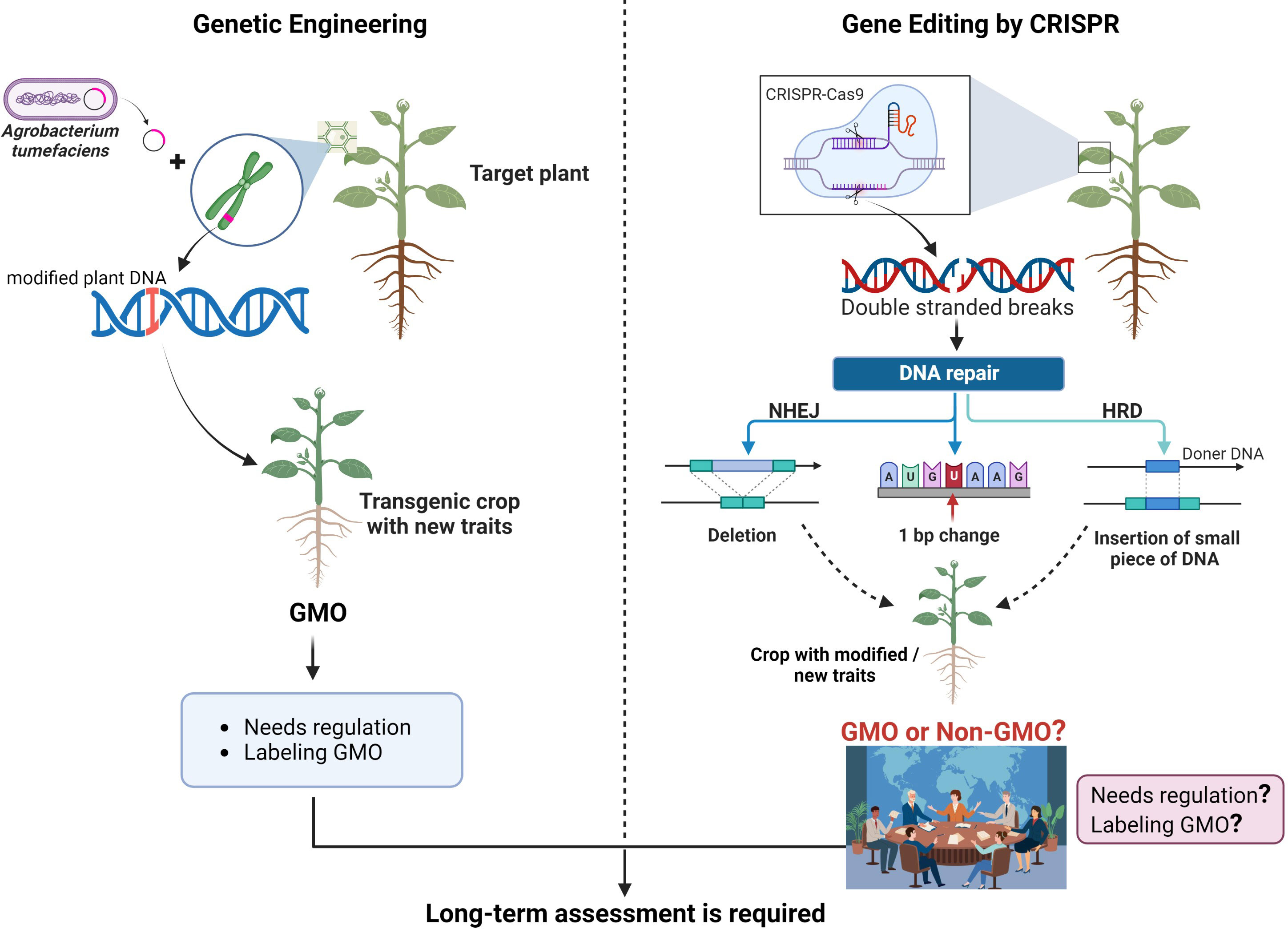
CRISPR is an innovative tool that is altering lives and making significant impacts in the medical sector. CRISPR, short for “Clustered Regularly Interspaced Short Palindromic Repeats,” is present in prokaryotes—tiny, single-celled organisms devoid of organelles. These sequences reside in the genomes of prokaryotes, which organize a cell’s DNA in clusters.
As a gene editing instrument, CRISPR is employed to alter specific DNA segments to combat severe illnesses. It is especially useful in protecting the body against viral attacks by integrating the foreign DNA into its genome, enabling it to identify and eliminate the virus in subsequent encounters. Notably, in contrast to other gene editing technologies, CRISPR is remarkably accurate and simpler to program, facilitating an easier process of sequence modification. While traditional gene-editing methods often utilize one protein, CRISPR employs RNA-guided targeting in conjunction with the Cas9 enzyme.
—
**How CRISPR Functions**
CRISPR operates by leveraging a natural defensive mechanism found in bacteria that allows them to detect and neutralize viral threats. When a virus infiltrates a bacterial cell (a prokaryote), the bacteria retain a fragment of the invader’s DNA within their own genome as a genetic “memory.” This capability allows the bacterium to recognize and react more efficiently to subsequent assaults.
In gene editing, this mechanism is modified using two essential elements: the Cas9 enzyme, which acts like molecular scissors to sever DNA, and guide RNA, which directs Cas9 to the particular genetic sequence requiring modification. After the designated DNA is cleaved, the cell’s intrinsic repair systems step in, enabling researchers to make alterations to the genetic framework.
Unlike previous instruments that depended on difficult-to-reprogram proteins, CRISPR’s RNA-guided mechanism is more versatile, simpler to develop, and extremely exact. This ease and precision have permitted CRISPR to be utilized in medicine, agriculture, manufacturing, and microbiology—such as engineering microbes to enhance product yields. However, as this article discusses, with increasing capabilities arise growing ethical dilemmas, especially concerning germline editing and genetic enhancement.
—
**First Patient to Receive a Personalized CRISPR Treatment**
In February 2025, the first personalized CRISPR treatment was administered for a baby named KJ to address a deficit in Carbamoyl Phosphate Synthetase 1 (CPS1)—an enzyme vital for converting ammonia (generated during protein metabolism) into urea. A team spearheaded by Dr. Rebecca Ahrens-Nicklas and Dr. Kiran Musunuru at the Children’s Hospital of Philadelphia devised this treatment following years of profound research in gene editing and collaboration with other healthcare professionals.
Their research concentrated on disorders impacting the urea cycle, which results in toxic ammonia accumulation, harming organs like the brain and liver. They customized the treatment specifically for KJ’s variant of CPS1 deficiency using preclinical studies on analogous mutations.
To date, the only CRISPR treatments sanctioned by the U.S. FDA have been for more prevalent conditions like sickle cell disease and beta thalassemia, affecting tens of thousands of patients. In KJ’s situation, his treatment was developed within six months of his birth, targeting his unique CPS1 variant. The team formulated a base editing therapy administered via lipid nanoparticles to his liver to rectify the defective enzyme.
The February treatment constituted the first of three doses; KJ received the subsequent two in March and April 2025. As of his final dose, he has not faced any major side effects, exhibits increased tolerance to dietary proteins, and requires reduced medication to regulate ammonia levels. Although ongoing monitoring will be necessary, Ahrens-Nicklas indicates that the outcomes thus far are encouraging.
—
**Ethical Issues Surrounding CRISPR**
As with any transformative technology, CRISPR prompts intricate ethical dilemmas. While its main intention is to modify somatic cells to remedy diseases, it can also be applied to gametes, trespassing into the contentious domain of germline editing. Modifying DNA that will be passed down to future generations is frequently regarded as unethical—especially when aimed at enhancing traits instead of addressing medical conditions.
In light of these issues, researchers have currently banned germline editing until the ethical and societal ramifications are further comprehended. The unpredictability of genetic alterations and their permanence across generations raises vital questions about where to set boundaries. What started as a pioneering idea has transformed into a formidable reality, inciting discussions on who has the authority to establish its limits.
CRISPR’s high costs and sophisticated infrastructure needs render it more accessible in affluent, developed countries—particularly in the West. As treatments remain high-priced, access is limited to those with means, exacerbating socioeconomic inequality. The likelihood of employing CRISPR to engineer so-called