Approximately one-third of individuals across the globe currently hosts a parasite in their brain. The majority will remain unaware of its presence. The single-celled organism Toxoplasma gondii, typically contracted from cats or undercooked meats, has perfected the technique of establishing long-term residence within human tissues. In healthy hosts, it stays dormant; however, in those with compromised immune systems, it can become lethal, causing severe brain inflammation.
Researchers affiliated with the University of Virginia School of Medicine have recently identified the reason this parasite typically remains controlled, revealing a counterintuitive strategy: immune cells dispatched to eliminate the invader can themselves become infected. Once this occurs, instead of continuing the fight, they undergo self-destruction, which also eliminates the parasite.
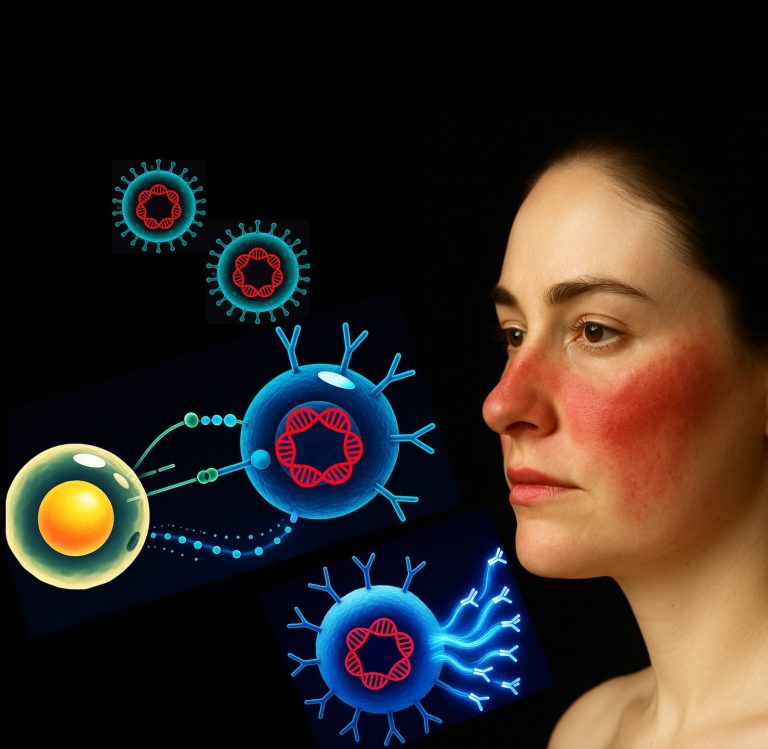
The results, reported in Science Advances, focus on CD8+ T cells, specialized defenders of the immune system that survey tissues for infected cells. Although scientists have long recognized the importance of these cells in regulating Toxoplasma, the UVA research team discovered that the parasite occasionally infiltrates these defenders. Instead of offering a safe refuge, this breach initiates a self-destruct mechanism governed by an enzyme named caspase-8. Thus, the infected T cell perishes, and the parasite loses both its sanctuary and its capacity to replicate.
When Protection Becomes a Trap
This revelation stemmed from studies involving laboratory mice engineered to lack caspase-8 in their T cells. These mice exhibited vigorous immune responses, inundating their brains with T cells and generating the expected chemical signals. Nevertheless, they fell gravely ill and succumbed shortly after infection, in contrast to normal mice, which exhibited no symptoms.
Microscopic examinations revealed the underlying issue: T cells in the susceptible mice were filled with replicating parasites, with parasite levels eight times greater than in healthy counterparts. Lacking the ability to self-terminate, these immune cells had transformed into Trojan horses, providing Toxoplasma a safeguarded niche to proliferate and disseminate through brain tissues.
“We discovered that these very T cells can become infected, and, if that happens, they may choose to die,” states Tajie Harris, director of UVA’s Center for Brain Immunology and Glia. “Toxoplasma parasites must reside within cells, hence the death of the host cell signifies the end of the line for the parasite.”
To identify the regions where caspase-8 was most active, the team implemented a method known as MERFISH to visualize gene expression throughout entire brain slices. The findings revealed that infiltrating immune cells, especially CD8+ T cells, contained elevated levels of the enzyme’s genetic instructions. In contrast, many resident brain cells exhibited low baseline activity, suggesting that the body depends on mobile immune units equipped with their own “kill switches” to oversee susceptible areas.
Why So Few Parasites Live in Immune Cells
These discoveries elucidate a wider trend in infectious diseases. Very few pathogens successfully infect T cells, and Harris hypothesizes that caspase-8 could be the cause. The only organisms recognized to persist within these immune cells have developed tactics to disrupt the enzyme’s function, effectively neutralizing the self-destruct mechanism. Prior to this investigation, no link had been established between caspase-8 and the brain’s defenses specifically against Toxoplasma.
This research reshapes the scientific perspective on chronic infections. Control does not necessarily equate to the complete eradication of a pathogen. At times, it involves forcing the invader into a stalemate in which any attempt to exploit the immune system results in self-sabotage. What initially seems to be a vulnerability in the defense framework reveals itself to be a calculated trap.
For individuals with healthy immune systems, this scorched-earth strategy operates quietly in the background, sustaining a precarious balance with a parasite that has co-evolved with humans for millennia. Gaining insights into this mechanism may ultimately yield therapies for immunocompromised patients, where the scale tips toward unrestrained infection. However, the study also prompts inquiries regarding the consequences of malfunctions in this cellular suicide process and whether certain chronic neurological disorders may stem from subtle breakdowns in this ancient defense system.
Science Advances: 10.1126/sciadv.adz4468
There’s no paywall here
If our reporting has informed or inspired you, please consider making a donation. Every contribution, regardless of size, enables us to continue providing accurate, engaging, and credible science and medical news. Independent journalism requires time, effort, and resources—your support ensures we can keep uncovering the stories that matter most to you.
Join us in making knowledge accessible and impactful. Thank you for standing with us!