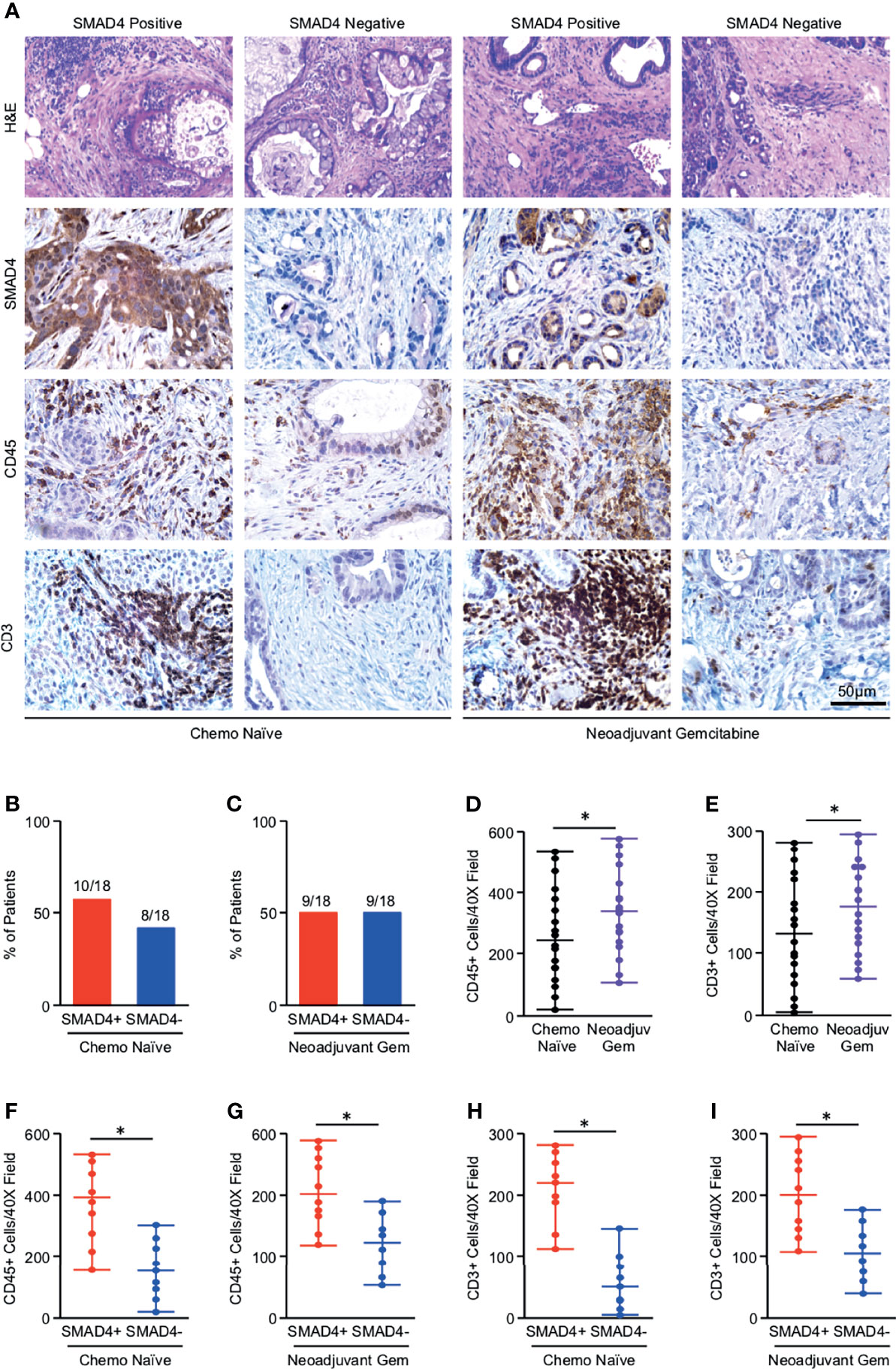
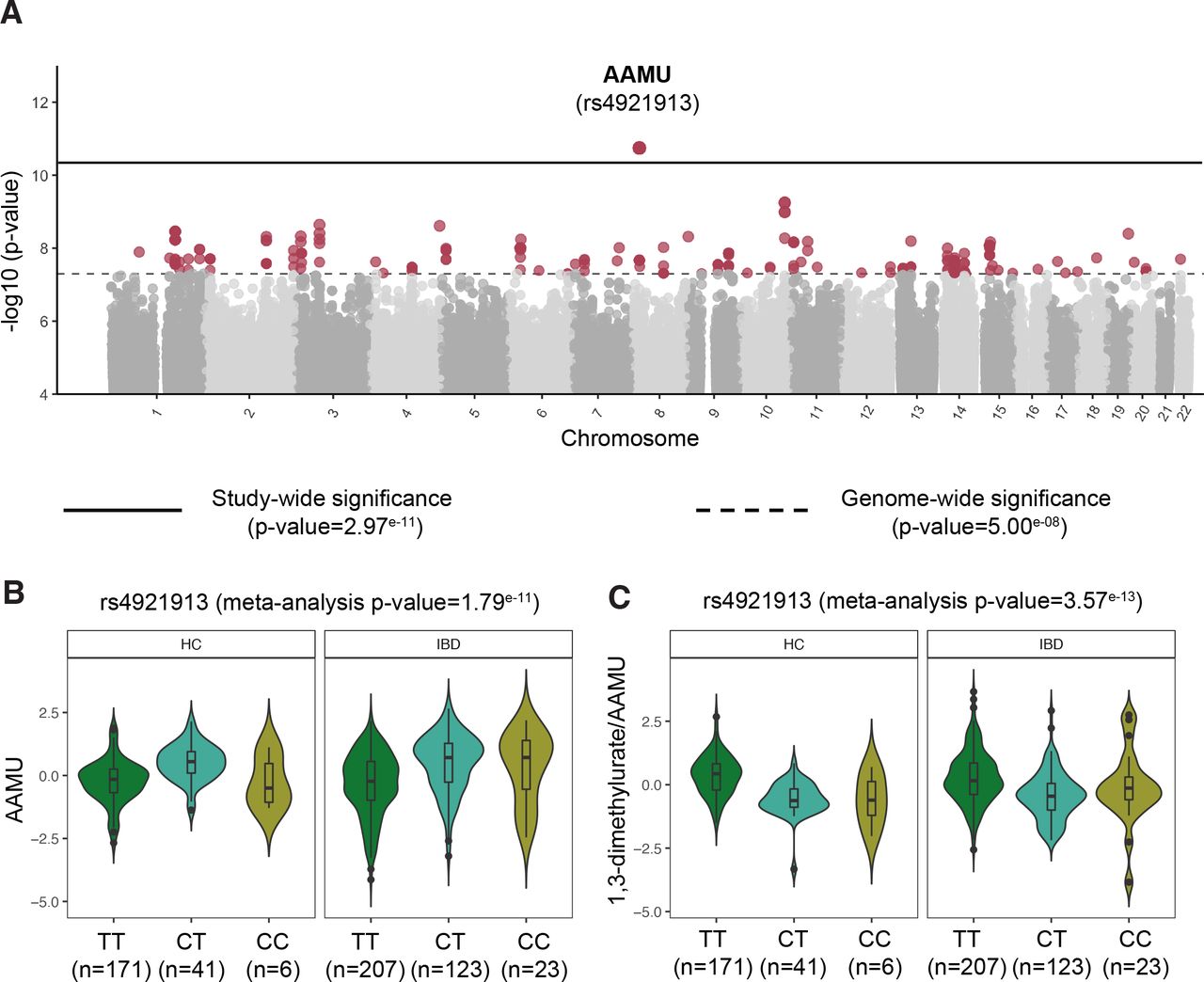
**Recent Discoveries in Diabetes Treatment: An Unexpected Defender Against Dementia** Individuals using a lesser-known category of diabetes medications are experiencing significantly lower dementia rates compared to those on traditional treatments. This finding, derived from a study monitoring over 450,000 Type 2 diabetes patients, indicates that DPP-4 inhibitors – medications that most individuals are unfamiliar with […]
Read More