A novel biocatalytic pathway has been developed that transforms aldehydes directly into amides – crucial components for numerous drug compounds. This innovative method, utilizing oxygen from the atmosphere and water as the solvent, has been employed to redesign synthetic routes for five drug molecules, showcasing its potential to provide a more sustainable, eco-friendly, and efficient solution for drug synthesis.
Amide bonds are prevalent in pharmaceuticals due to their chemical stability, ease of formation, and biocompatibility. They play a significant role in regulating characteristics such as solubility, shape, and a molecule’s interactions with proteins in the human body. While an amide bond by itself does not classify a compound as a drug, it frequently serves as a vital structural feature balancing a molecule’s stability with its biological activity.
Typically, amide bonds are created through chemical synthesis by linking carboxylic acids – which can be sourced from aldehydes or alcohols – with amines. However, this process necessitates high-activation-state precursors from acids, protective group strategies, toxic coupling reagents, transition metal catalysts, and considerable quantities of organic solvents, leading to waste generation and increased energy consumption. In recent years, more eco-friendly, enzymatic approaches using ATP-dependent ligases have been developed; however, these methods still rely on carboxylic acid precursors.
Now, Xiaoguang Lei and his team at Peking University, China, have created an innovative biocatalytic and ‘low activation’ pathway that entirely circumvents the need for carboxylic acids, directly synthesizing amide bonds from aldehydes or, via a two-step process, from alcohols.
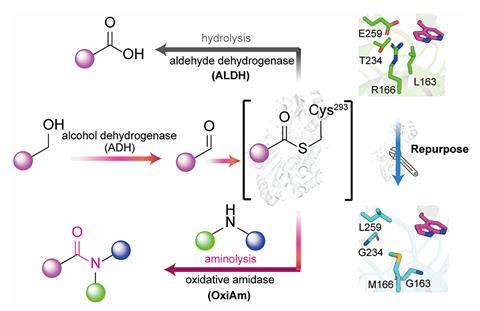
‘We began by investigating whether we could persuade an enzyme that typically converts aldehydes into carboxylic acids to take on a different task,’ explains Lei. ‘In its standard reaction, this enzyme, aldehyde dehydrogenase, temporarily produces a highly reactive intermediate. We sought to determine if we could modify the enzyme so that instead of reacting with water to generate an acid, the intermediate would interact with an amine to yield an amide.’
To achieve this, the group employed protein engineering methods to reform the active site of aldehyde dehydrogenase, creating enzymes they named oxidative amidases. When reacting with an aldehyde, the active site facilitated the entry of an amine to engage with the intermediate rather than water, enabling direct amide production. When starting from alcohols, an initial enzyme converted the alcohol to an aldehyde before following the same pathway.
Lei notes that the primary challenge lay in preventing water from reacting with the intermediate within the enzyme while promoting an amine to outcompete water and effectively ‘hijack’ the intermediate. To address this, the team adjusted the pH to approximately 10, ensuring the amine remained nucleophilic enough. Additionally, hydrophilic residues in the native enzyme were replaced with water-repelling ones.
‘Achieving this necessitated meticulous structural analysis and precise redesign of the enzyme’s active site to manipulate both spatial arrangement and chemical environment,’ states Lei. ‘One unexpected outcome was the minimal changes required to completely redirect the enzyme’s chemistry. Only four targeted mutations were sufficient to transition the primary product from an acid to an amide.’
Findings indicated that this system was effective across various aldehydes and amines, including those pertinent to pharmaceuticals. As a proof-of-concept, the researchers showcased that the oxidative amidases could facilitate the production of five key pharmaceuticals, including drugs intended for treating anemia and leukemia.
‘The remarkable enzyme engineering accomplished by Lei and colleagues introduces a new biocatalytic pathway to amides, initiated from amine and aldehyde precursors,’ comments Jason Mickelfield, who researches biocatalysis at Imperial College, London. ‘Any innovative biocatalytic alternative is important, and I am eager to determine whether this method demonstrates the same versatility as previous ATP-dependent ligase strategies.’
‘Our long-term aspiration is that this chemistry will enable chemists to devise drug synthesis pathways in entirely new manners, utilizing simpler and more readily accessible building blocks like alcohols,’ says Lei. ‘We are currently focused on broadening the spectrum of enzymes and substrates, enhancing efficiency, and investigating how this approach can be applied to pharmaceutical manufacturing on an industrial scale.’